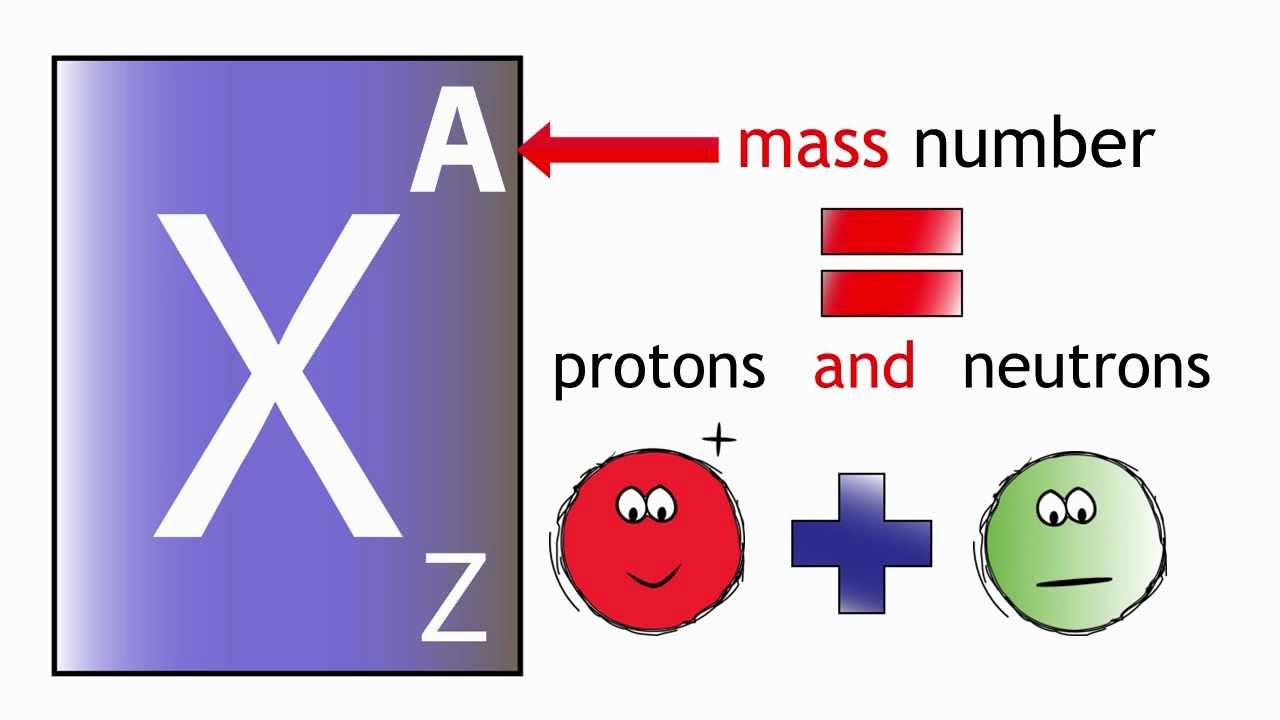
Tin Atomic Number Mass Number Protons Neutrons Electrons . Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For stable elements, there is usually a variety of stable isotopes. Therefore, a tin atom has. Determine the number of protons and electrons in an atom. Write and interpret symbols that depict the atomic number, mass. Therefore, we can subtract the number of protons from the. The difference between the neutron number and the atomic number is known as the neutron excess : Identify the charge and relative mass of subatomic particles. Determine the number of protons, neutrons, and electrons in an atom. Define atomic and mass numbers. From the periodic table, we find that it has 29 protons. Number of neutrons in tin. The mass number (65) is the sum of the number of protons and neutrons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. We know that the atomic number of tin is 50 and the atomic average mass number is about 119.
from periodictable.me
From the periodic table, we find that it has 29 protons. Number of neutrons in tin. Determine the number of protons and electrons in an atom. Write and interpret symbols that depict the atomic number, mass. The mass number (65) is the sum of the number of protons and neutrons. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Determine the number of protons, neutrons, and electrons in an atom. Identify the charge and relative mass of subatomic particles. Neutron number plus atomic number equals atomic mass number:
Atomic Number Examples Archives Dynamic Periodic Table of Elements
Tin Atomic Number Mass Number Protons Neutrons Electrons We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Neutron number plus atomic number equals atomic mass number: The difference between the neutron number and the atomic number is known as the neutron excess : Determine the number of protons, neutrons, and electrons in an atom. The mass number (65) is the sum of the number of protons and neutrons. Determine the number of protons and electrons in an atom. From the periodic table, we find that it has 29 protons. Therefore, a tin atom has. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Therefore, we can subtract the number of protons from the. Write and interpret symbols that depict the atomic number, mass. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Number of neutrons in tin. For stable elements, there is usually a variety of stable isotopes. Identify the charge and relative mass of subatomic particles.
From stock.adobe.com
Representation of an atom. Atoms and elements. Symbol of element, mass Tin Atomic Number Mass Number Protons Neutrons Electrons The mass number (65) is the sum of the number of protons and neutrons. Therefore, we can subtract the number of protons from the. Write and interpret symbols that depict the atomic number, mass. Neutron number plus atomic number equals atomic mass number: 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.transformationtutoring.com
Particles Inside An Atom Protons, Neutrons and Electrons Guide As Well Tin Atomic Number Mass Number Protons Neutrons Electrons Define atomic and mass numbers. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. From the periodic table, we find that it has 29 protons. For stable elements, there is usually a variety of stable isotopes. Number of neutrons in tin. The difference between the neutron number and the atomic. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From brainly.ph
Complete the table below, write the atomic number, number of protons Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. Therefore, we can subtract the number of protons from the. Define atomic and mass numbers. Determine the number of protons and electrons in an atom. From the periodic table, we find that it has 29 protons. The mass number (65) is the sum of the number of protons and. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.pinterest.com
3 Ways to Calculate Atomic Mass wikiHow Teaching chemistry Tin Atomic Number Mass Number Protons Neutrons Electrons Write and interpret symbols that depict the atomic number, mass. Neutron number plus atomic number equals atomic mass number: We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Identify the charge and relative mass of subatomic particles. Define atomic and mass numbers. Determine the number of protons, neutrons, and electrons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From periodictable.me
Periodic Table Element With Atomic Mass And Atomic Number Tin Atomic Number Mass Number Protons Neutrons Electrons From the periodic table, we find that it has 29 protons. Therefore, we can subtract the number of protons from the. Neutron number plus atomic number equals atomic mass number: Number of neutrons in tin. Therefore, a tin atom has. The mass number (65) is the sum of the number of protons and neutrons. The difference between the neutron number. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From periodictable.me
Atomic Number Examples Archives Dynamic Periodic Table of Elements Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. Therefore, a tin atom has. Identify the charge and relative mass of subatomic particles. Write and interpret symbols that depict the atomic number, mass. Determine the number of protons and electrons in an atom. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From milonathan.blogspot.com
Atomic Number Of Atom Tin Atomic Number Mass Number Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Identify the charge and relative mass of subatomic particles. From the periodic table, we find that it has 29 protons. Neutron number plus atomic number equals atomic mass number: The mass number (65) is the sum of the. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Tin Atomic Number Mass Number Protons Neutrons Electrons Identify the charge and relative mass of subatomic particles. Therefore, we can subtract the number of protons from the. Number of neutrons in tin. The difference between the neutron number and the atomic number is known as the neutron excess : Neutron number plus atomic number equals atomic mass number: We know that the atomic number of tin is 50. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.wikihow.com
How to Find the Number of Neutrons in an Atom 11 Steps Tin Atomic Number Mass Number Protons Neutrons Electrons From the periodic table, we find that it has 29 protons. For stable elements, there is usually a variety of stable isotopes. Therefore, a tin atom has. Identify the charge and relative mass of subatomic particles. The difference between the neutron number and the atomic number is known as the neutron excess : The mass number (65) is the sum. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.javatpoint.com
Definition of Atomic Number JavaTpoint Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. For stable elements, there is usually a variety of stable isotopes. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. The mass number (65) is the sum of the number of protons and neutrons. Identify the. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From findstuffsonline.com
How To Find The Protons Neutrons And Electrons Of An Element On The Tin Atomic Number Mass Number Protons Neutrons Electrons Define atomic and mass numbers. Number of neutrons in tin. Therefore, a tin atom has. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Identify the charge and relative mass of subatomic particles. Determine the number of protons and electrons in an atom. Therefore, we can subtract the number of. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.slideserve.com
PPT Part A Atomic Structure PowerPoint Presentation, free download Tin Atomic Number Mass Number Protons Neutrons Electrons Identify the charge and relative mass of subatomic particles. Write and interpret symbols that depict the atomic number, mass. For stable elements, there is usually a variety of stable isotopes. The mass number (65) is the sum of the number of protons and neutrons. Number of neutrons in tin. The difference between the neutron number and the atomic number is. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.numerade.com
SOLVED 'given below are neutral atoms. determine the number of protons Tin Atomic Number Mass Number Protons Neutrons Electrons Neutron number plus atomic number equals atomic mass number: Determine the number of protons, neutrons, and electrons in an atom. The mass number (65) is the sum of the number of protons and neutrons. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Sources, facts, uses, scarcity (sri), podcasts, alchemical. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.wikihow.com
How to Find the Number of Protons, Neutrons, and Electrons Tin Atomic Number Mass Number Protons Neutrons Electrons The difference between the neutron number and the atomic number is known as the neutron excess : Define atomic and mass numbers. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Determine the number of protons and electrons in an atom. Therefore, we can subtract the number of protons from the. We know that the atomic number of tin is 50. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.alamy.com
Sn Tin Chemical Element Periodic Table. Single vector illustration Tin Atomic Number Mass Number Protons Neutrons Electrons The mass number (65) is the sum of the number of protons and neutrons. The difference between the neutron number and the atomic number is known as the neutron excess : We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Number of neutrons in tin. Sources, facts, uses, scarcity (sri),. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.youtube.com
Atomic Number and Mass Number.mov YouTube Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. For stable elements, there is usually a variety of stable isotopes. Define atomic and mass numbers. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. The difference between the neutron number and the atomic number is known as the. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.coscinecreative.com
How to Teach Atomic Number and Atomic Mass (So Students Remember it Tin Atomic Number Mass Number Protons Neutrons Electrons For stable elements, there is usually a variety of stable isotopes. Write and interpret symbols that depict the atomic number, mass. Therefore, a tin atom has. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Identify the charge and relative mass of subatomic particles. Neutron number plus. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.coscinecreative.com
How to Teach Finding Protons, Neutrons, and Electrons in an Element Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, we can subtract the number of protons from the. Define atomic and mass numbers. Identify the charge and relative mass of subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Neutron number plus atomic number equals atomic mass number: Determine the number of protons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.youtube.com
How to find the number of Protons, Neutrons and Electrons? Chemistry Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. For stable elements, there is usually a variety of stable isotopes. The mass number (65) is the sum of the number of protons and neutrons. Neutron number plus atomic number equals atomic mass number: 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From mmerevise.co.uk
Atoms Questions and Revision MME Tin Atomic Number Mass Number Protons Neutrons Electrons The difference between the neutron number and the atomic number is known as the neutron excess : Determine the number of protons, neutrons, and electrons in an atom. Neutron number plus atomic number equals atomic mass number: For stable elements, there is usually a variety of stable isotopes. Determine the number of protons and electrons in an atom. Therefore, we. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.nuclear-power.com
Tin Atomic Number Atomic Mass Density of Tin Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Define atomic and mass numbers. For stable elements, there is usually a variety of stable isotopes. Identify the charge and relative mass of subatomic particles. We know. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, a tin atom has. Determine the number of protons and electrons in an atom. Write and interpret symbols that depict the atomic number, mass. Determine the number of protons, neutrons, and electrons in an atom. Number of neutrons in tin. The mass number (65) is the sum of the number of protons and neutrons. For stable elements, there is. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From quizzlibmisty.z21.web.core.windows.net
How To Find The Protons Neutrons Electrons Tin Atomic Number Mass Number Protons Neutrons Electrons From the periodic table, we find that it has 29 protons. Neutron number plus atomic number equals atomic mass number: Write and interpret symbols that depict the atomic number, mass. For stable elements, there is usually a variety of stable isotopes. Identify the charge and relative mass of subatomic particles. The mass number (65) is the sum of the number. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From material-properties.org
Tin Periodic Table and Atomic Properties Tin Atomic Number Mass Number Protons Neutrons Electrons The difference between the neutron number and the atomic number is known as the neutron excess : Define atomic and mass numbers. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. From the periodic table, we find that it has 29 protons. Write and interpret symbols that depict the atomic. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.studypool.com
SOLUTION DPAGENCHEM1 Activity 02_Element, Atomic Number, Mass Number Tin Atomic Number Mass Number Protons Neutrons Electrons Number of neutrons in tin. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Therefore, a tin atom has. Define atomic and mass numbers. The difference between the neutron number and the atomic number is known as the neutron excess : Neutron number plus atomic number equals atomic mass number:. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From utedzz.blogspot.com
Periodic Table Numbers Of Neutrons Protons And Electrons Periodic Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, a tin atom has. Identify the charge and relative mass of subatomic particles. Determine the number of protons and electrons in an atom. The difference between the neutron number and the atomic number is known as the neutron excess : For stable elements, there is usually a variety of stable isotopes. Determine the number of protons, neutrons, and electrons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From slideplayer.com
Atomic Symbols = = mass atomic protons + neutrons protons ppt Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, a tin atom has. We know that the atomic number of tin is 50 and the atomic average mass number is about 119. Write and interpret symbols that depict the atomic number, mass. Define atomic and mass numbers. Determine the number of protons and electrons in an atom. The mass number (65) is the sum of the number of. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.numerade.com
SOLVED Please fill out correctly 15 POINTS Part 2 Complete the chart Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons, neutrons, and electrons in an atom. Number of neutrons in tin. The difference between the neutron number and the atomic number is known as the neutron excess : Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. The mass number (65) is the sum of the number of protons and neutrons. Determine the number of protons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From quizlet.com
Protons, Neutrons, Electrons and Atomic Number, Mass Number Diagram Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, a tin atom has. From the periodic table, we find that it has 29 protons. Determine the number of protons and electrons in an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Determine the number of protons, neutrons, and electrons in an atom. Neutron number plus atomic number equals atomic mass number: The difference between the neutron number. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Sn (Tin Tin Atomic Number Mass Number Protons Neutrons Electrons 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Identify the charge and relative mass of subatomic particles. Neutron number plus atomic number equals atomic mass number: The mass number (65) is the sum of the number of protons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.numerade.com
SOLVED Atomic Number Number of Protons Number of Neutrons Number of Tin Atomic Number Mass Number Protons Neutrons Electrons Determine the number of protons and electrons in an atom. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Therefore, a tin atom has. For stable elements, there is usually a variety of stable isotopes. Identify the charge and relative mass of subatomic particles. From the periodic table, we find that it has 29 protons. 112 rows protons, neutrons and electrons. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From utedzz.blogspot.com
Rounded Periodic Table With Mass And Atomic Number Periodic Table Tin Atomic Number Mass Number Protons Neutrons Electrons Therefore, we can subtract the number of protons from the. Identify the charge and relative mass of subatomic particles. The mass number (65) is the sum of the number of protons and neutrons. Neutron number plus atomic number equals atomic mass number: For stable elements, there is usually a variety of stable isotopes. The difference between the neutron number and. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID4825731 Tin Atomic Number Mass Number Protons Neutrons Electrons Identify the charge and relative mass of subatomic particles. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below (you will get the list + shell. Therefore, we can subtract the number of protons from the. For stable elements, there is usually a variety of stable isotopes. Therefore, a tin atom has. We know that. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From mungfali.com
Protons Neutrons And Electrons Symbols Tin Atomic Number Mass Number Protons Neutrons Electrons Define atomic and mass numbers. The mass number (65) is the sum of the number of protons and neutrons. Number of neutrons in tin. Therefore, a tin atom has. Write and interpret symbols that depict the atomic number, mass. Determine the number of protons, neutrons, and electrons in an atom. Neutron number plus atomic number equals atomic mass number: Sources,. Tin Atomic Number Mass Number Protons Neutrons Electrons.
From sciencenotes.org
What Is an Atomic Number? Definition and Examples Tin Atomic Number Mass Number Protons Neutrons Electrons Define atomic and mass numbers. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For stable elements, there is usually a variety of stable isotopes. Write and interpret symbols that depict the atomic number, mass. From the periodic table, we find that it has 29 protons. 112 rows protons, neutrons and electrons of all elements are mentioned in the table below. Tin Atomic Number Mass Number Protons Neutrons Electrons.