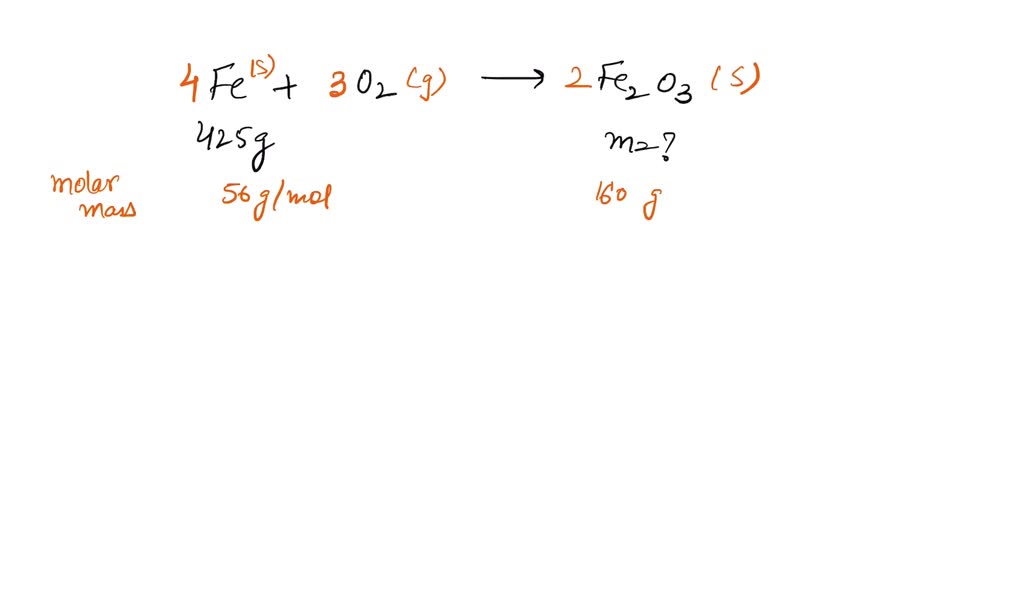Iron (Iii) Charge . So fe +2 is iron(ii) and fe +3 is iron(iii). Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. 93 rows there are four ways to find the charge of an element: The usual charge of an element is common to its group. The ferrous cation (fe2+) and ferric. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Iron exists in two oxidation states:
from www.numerade.com
Iron can form two possible ions, but the ion with a 3+ charge is specified here. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). The ferrous cation (fe2+) and ferric. Iron exists in two oxidation states: Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. The usual charge of an element is common to its group. The oxide has a 2− charge as an ion. 93 rows there are four ways to find the charge of an element: Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. So fe +2 is iron(ii) and fe +3 is iron(iii).
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a
Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. The oxide has a 2− charge as an ion. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. The ferrous cation (fe2+) and ferric. Iron can form two possible ions, but the ion with a 3+ charge is specified here. So fe +2 is iron(ii) and fe +3 is iron(iii). 93 rows there are four ways to find the charge of an element: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Iron exists in two oxidation states: The usual charge of an element is common to its group.
From exodawtpi.blob.core.windows.net
What Does Fe Stand For In Iron at Charles Marrero blog Iron (Iii) Charge The ferrous cation (fe2+) and ferric. Iron exists in two oxidation states: Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. So fe +2 is iron(ii) and fe +3 is iron(iii). For example, knowing that iron can have a +2 or +3. Iron (Iii) Charge.
From www.youtube.com
How to Write the Formula for Iron (III) phosphate YouTube Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). 93 rows there are four ways to find the charge of an element: The oxide has a 2− charge as an ion. The ferrous cation (fe2+) and ferric. The usual. Iron (Iii) Charge.
From www.fishersci.com
Iron(III) bromide, anhydrous, 98+, Thermo Scientific Chemicals Iron (Iii) Charge Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. Iron can form two possible ions, but the ion with a 3+ charge is specified here. 93 rows there are four ways to find the charge of an element: Manganese(iii) mn3+ 4+ tin(iv). Iron (Iii) Charge.
From ceukyrig.blob.core.windows.net
Aqueous Zinc Bromide Equation at Heather Spiers blog Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Iron can form two possible ions, but the ion with a 3+ charge is specified here. The. Iron (Iii) Charge.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron (Iii) Charge Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. So fe +2 is iron(ii) and fe +3 is iron(iii). The oxide has a 2− charge as an ion. The usual charge of an element is common to its group. Some of the metals form very common ions which have latin names. Iron (Iii) Charge.
From www.slideserve.com
PPT A2 Chemistry transition metal complexes a visual guide PowerPoint Iron (Iii) Charge Iron exists in two oxidation states: Iron can form two possible ions, but the ion with a 3+ charge is specified here. The usual charge of an element is common to its group. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the.. Iron (Iii) Charge.
From www.youtube.com
How to Write the Formula for Iron (III) chloride YouTube Iron (Iii) Charge Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. 93 rows there are four ways to find the charge of an element: The usual charge of an element is common to its group. The oxide has a 2− charge as an ion.. Iron (Iii) Charge.
From www.numerade.com
SOLVED of iron with oxygen gas and water produces iron (III) oxide (a Iron (Iii) Charge The usual charge of an element is common to its group. The ferrous cation (fe2+) and ferric. Iron can form two possible ions, but the ion with a 3+ charge is specified here. For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o. Iron (Iii) Charge.
From davis-well-rodgers.blogspot.com
Iron Iii Chloride and Sodium Hydroxide Ionic Equation DaviswellRodgers Iron (Iii) Charge The oxide has a 2− charge as an ion. The ferrous cation (fe2+) and ferric. 93 rows there are four ways to find the charge of an element: So fe +2 is iron(ii) and fe +3 is iron(iii). Some of the metals form very common ions which have latin names that are in common use, and you need to be. Iron (Iii) Charge.
From klaxfvotc.blob.core.windows.net
What Is Irons Charge In Iron (Ii) Phosphate at Ha Lumpkin blog Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). So fe +2 is iron(ii) and fe +3 is iron(iii). Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. Some of. Iron (Iii) Charge.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog Iron (Iii) Charge Iron exists in two oxidation states: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with. Iron (Iii) Charge.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron (Iii) Charge Iron can form two possible ions, but the ion with a 3+ charge is specified here. The usual charge of an element is common to its group. The ferrous cation (fe2+) and ferric. Iron exists in two oxidation states: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo. Iron (Iii) Charge.
From www.scribd.com
4 Changes of Iron(II) Ions to Iron(III) and Vice Versa Redox Iron Iron (Iii) Charge The ferrous cation (fe2+) and ferric. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. The oxide has a 2− charge as an ion. So fe +2 is iron(ii) and fe +3 is iron(iii). 93. Iron (Iii) Charge.
From www.youtube.com
Formation of Rust Hydrated Iron (III) Oxide YouTube Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: Iron can form two possible ions, but the ion with a 3+ charge is specified here. The usual charge of an element is common to its group. So fe +2 is iron(ii) and fe +3 is iron(iii). For example, knowing that iron can have a +2 or. Iron (Iii) Charge.
From www.nagwa.com
Question Video Deducing the Ionic Formula of an Ionic Compound Where Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Iron can form two possible ions, but the ion with a 3+ charge is specified here. The usual charge of an element is common to its group. Some of the. Iron (Iii) Charge.
From www.showme.com
Write the formula for iron (III) oxide and iron (III) sulfate Iron (Iii) Charge Iron exists in two oxidation states: Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. So fe +2 is iron(ii) and fe +3 is iron(iii). The oxide has a 2− charge as an ion. The usual charge of an element is common to its group. Some of the metals form very. Iron (Iii) Charge.
From www.youtube.com
Is Fe2O3, Iron (III) oxide. Ionic or Covalent? YouTube Iron (Iii) Charge The usual charge of an element is common to its group. 93 rows there are four ways to find the charge of an element: The oxide has a 2− charge as an ion. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the.. Iron (Iii) Charge.
From www.indiamart.com
Iron 3 Hydroxide Polymaltose at Rs 1050/kg Active Pharmaceutical Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: The oxide has a 2− charge as an ion. The ferrous cation (fe2+) and ferric. So fe +2 is iron(ii) and fe +3 is iron(iii). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Some of the metals form very. Iron (Iii) Charge.
From www.askdifference.com
Iron II Chloride vs. Iron III Chloride — What’s the Difference? Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: Iron exists in two oxidation states: The oxide has a 2− charge as an ion. The ferrous cation (fe2+) and ferric. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. For example, knowing that iron can have a. Iron (Iii) Charge.
From streetlink.org.uk
💐 Synthesis of iron oxalate complex. Chem 7L Expt 4 Synthesis and Iron (Iii) Charge Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. So fe +2 is iron(ii) and fe +3 is iron(iii). The ferrous cation (fe2+) and ferric. 93 rows there are four ways to find the charge of an element: Manganese(iii) mn3+ 4+ tin(iv). Iron (Iii) Charge.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog Iron (Iii) Charge Iron can form two possible ions, but the ion with a 3+ charge is specified here. 93 rows there are four ways to find the charge of an element: The usual charge of an element is common to its group. Some of the metals form very common ions which have latin names that are in common use, and you need. Iron (Iii) Charge.
From app.pandai.org
Chemical Formulae Iron (Iii) Charge Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. So fe +2 is iron(ii) and fe +3 is iron(iii). The oxide has a 2− charge as an ion. 93 rows there are four ways to find the charge of an element: The. Iron (Iii) Charge.
From www.difference.wiki
Iron II Chloride vs. Iron III Chloride What’s the Difference? Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). 93 rows there are four ways to find the charge of an element: Some of the metals form very common ions which have latin names that are in common use,. Iron (Iii) Charge.
From www.youtube.com
How to Balance Fe + S = FeS (Iron + Sulfur) YouTube Iron (Iii) Charge Iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxide has a 2− charge as an ion. The ferrous cation (fe2+) and ferric. Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. Iron exists. Iron (Iii) Charge.
From www.slideserve.com
PPT Chemical Nomenclature (Naming Compounds) PowerPoint Presentation Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). So fe +2 is iron(ii) and fe +3 is iron(iii). Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. Iron exists. Iron (Iii) Charge.
From socratic.org
In a Stock system name such as iron (III) sulfate, what do the Roman Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: So fe +2 is iron(ii) and fe +3 is iron(iii). Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman. Iron (Iii) Charge.
From achs-prod.acs.org
Iron(II) and Iron(III) Spin Crossover Toward an Optimal Density Iron (Iii) Charge So fe +2 is iron(ii) and fe +3 is iron(iii). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Iron exists in two oxidation states: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3. Iron (Iii) Charge.
From www.youtube.com
Lewis Structure of Fe2O3, Iron (III) Oxide YouTube Iron (Iii) Charge Iron can form two possible ions, but the ion with a 3+ charge is specified here. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. So fe +2 is iron(ii) and fe +3 is iron(iii). 93 rows there are four ways to find the charge of an element: Some of the. Iron (Iii) Charge.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). The oxide has a 2− charge as an ion. 93 rows there are four ways to find the charge of an element: Iron can form two possible ions, but the. Iron (Iii) Charge.
From testbook.com
Iron (III) Hydroxide Formula Its Structure, Preparation & Uses Iron (Iii) Charge 93 rows there are four ways to find the charge of an element: For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). The usual charge of an element is common to its group. The ferrous cation (fe2+) and ferric.. Iron (Iii) Charge.
From hdhg2022.en.made-in-china.com
Fecl3 Iron (III) Chloride China Ferric Chloride Anhydrous and Fecl3 Iron (Iii) Charge The usual charge of an element is common to its group. The oxide has a 2− charge as an ion. Iron can form two possible ions, but the ion with a 3+ charge is specified here. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. The ferrous cation (fe2+) and ferric.. Iron (Iii) Charge.
From www.chegg.com
Solved Iron reacts with oxygen at high temperatures to form Iron (Iii) Charge Some of the metals form very common ions which have latin names that are in common use, and you need to be familiar with those in the. So fe +2 is iron(ii) and fe +3 is iron(iii). The ferrous cation (fe2+) and ferric. The usual charge of an element is common to its group. Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv). Iron (Iii) Charge.
From slideplayer.com
Ionic Compounds Naming ppt download Iron (Iii) Charge So fe +2 is iron(ii) and fe +3 is iron(iii). For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). Iron can form two possible ions, but the ion with a 3+ charge is specified here. Iron exists in two. Iron (Iii) Charge.
From joigjogsq.blob.core.windows.net
Iron Iii Oxide Negative Ion at Emily Gaines blog Iron (Iii) Charge For example, knowing that iron can have a +2 or +3 charge can help predict the formation of compounds like feo (iron(ii) oxide) or fe 2 o 3 (iron(iii) oxide). 93 rows there are four ways to find the charge of an element: So fe +2 is iron(ii) and fe +3 is iron(iii). The usual charge of an element is. Iron (Iii) Charge.
From slideplayer.com
Chapter 7 Compounds and Their Bonds ppt download Iron (Iii) Charge Manganese(iii) mn3+ 4+ tin(iv) sn4+ nickel(iv) ni4+ lead(iv) pb4+ roman numeral notation indicates charge of ion when element. The usual charge of an element is common to its group. Iron exists in two oxidation states: So fe +2 is iron(ii) and fe +3 is iron(iii). 93 rows there are four ways to find the charge of an element: For example,. Iron (Iii) Charge.