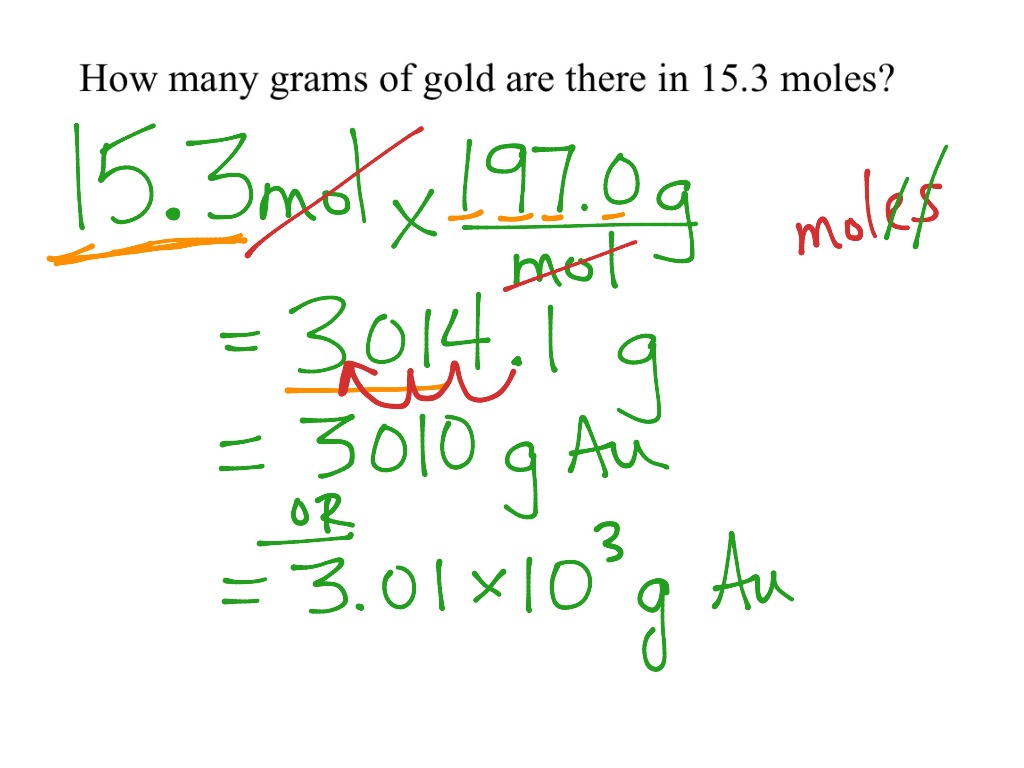Moles Chemistry Real Life Examples . In chemistry, the mole is a unit used to talk about atoms. 6.02214 x 1023, the number of units in a mole. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. These numbers are very important for telling us about the quantity of elements. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. National chemistry week (ncw) mole day. It is similar to other units we use everyday. This very large number is called avogadro’s number: The mole is a key unit in chemistry.
from www.showme.com
In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. These numbers are very important for telling us about the quantity of elements. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. In chemistry, the mole is a unit used to talk about atoms. The mole is a key unit in chemistry. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. This very large number is called avogadro’s number: National chemistry week (ncw) mole day. It is similar to other units we use everyday.
Mole calculations CHEM 1 Science, Chemistry, Moles ShowMe
Moles Chemistry Real Life Examples The mole is a key unit in chemistry. National chemistry week (ncw) mole day. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. This very large number is called avogadro’s number: In chemistry, the mole is a unit used to talk about atoms. These numbers are very important for telling us about the quantity of elements. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. 6.02214 x 1023, the number of units in a mole. It is similar to other units we use everyday. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. The mole is a key unit in chemistry. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs.
From sciencetrends.com
How To Convert Moles To Molecules With Examples Science Trends Moles Chemistry Real Life Examples In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. This very large number. Moles Chemistry Real Life Examples.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free Moles Chemistry Real Life Examples Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. These numbers are very important for telling us about the quantity of elements. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. National chemistry week (ncw) mole day. The mole, or “mol”. Moles Chemistry Real Life Examples.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube Moles Chemistry Real Life Examples The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. This very large number is called avogadro’s number: In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. 6.02214 x 1023, the number of units in a mole. The mole, or “mol” is. Moles Chemistry Real Life Examples.
From sciencenotes.org
Moles to Grams Conversion Examples Moles Chemistry Real Life Examples The mole is a key unit in chemistry. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in. Moles Chemistry Real Life Examples.
From mungfali.com
The Mole Chemistry Moles Chemistry Real Life Examples The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. The mole is a key unit in chemistry. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup). Moles Chemistry Real Life Examples.
From thescienceteacher.co.uk
Amounts of substance teaching resources the science teacher Moles Chemistry Real Life Examples The mole is a key unit in chemistry. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. These numbers are very important for telling us about the. Moles Chemistry Real Life Examples.
From www.youtube.com
How To Convert Moles to Grams YouTube Moles Chemistry Real Life Examples The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. National chemistry week (ncw) mole day. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. The mole, or “mol” is a unit of measurement in. Moles Chemistry Real Life Examples.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation Moles Chemistry Real Life Examples Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. In chemistry, the mole is a unit used to talk about atoms. These numbers are very important for telling us about the quantity of elements. It is similar to other units we use everyday. The mole is a key unit in. Moles Chemistry Real Life Examples.
From general.chemistrysteps.com
How To Convert Grams To Moles Chemistry Steps Moles Chemistry Real Life Examples 6.02214 x 1023, the number of units in a mole. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. These numbers are very important for telling us about the quantity of elements. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities. Moles Chemistry Real Life Examples.
From cemhytuq.blob.core.windows.net
How Is Physical Chemistry Used In Everyday Life at Teresa Santiago blog Moles Chemistry Real Life Examples Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. This very large number is called avogadro’s number: 6.02214 x 1023, the number of units in a mole. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. It is similar. Moles Chemistry Real Life Examples.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles Moles Chemistry Real Life Examples Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. In chemistry, the mole is a unit used to talk about atoms. This very large number is called avogadro’s number: 6.02214 x 1023, the number of units in a mole. These numbers are very important for telling us about the quantity of. Moles Chemistry Real Life Examples.
From www.slideserve.com
PPT Moles, Mathematics of Chemistry PowerPoint Presentation, free Moles Chemistry Real Life Examples The mole is a key unit in chemistry. National chemistry week (ncw) mole day. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. These numbers are very important for telling us about the quantity of elements. The molar mass of a substance, in grams, is numerically. Moles Chemistry Real Life Examples.
From www.youtube.com
What Is a Mole? Chemistry Matters YouTube Moles Chemistry Real Life Examples It is similar to other units we use everyday. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. 6.02214 x 1023, the number of units in a mole. In chemistry, the mole is a unit used to talk about atoms. This very large number is called avogadro’s number: In chemistry, moles are a. Moles Chemistry Real Life Examples.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles To Grams Moles Chemistry Real Life Examples It is similar to other units we use everyday. The mole is a key unit in chemistry. National chemistry week (ncw) mole day. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. 6.02214 x 1023, the number of units in a mole. Mole, standard unit (6.02214076. Moles Chemistry Real Life Examples.
From www.youtube.com
Moles for AQA 91 GCSE Chemistry and Trilogy Science) YouTube Moles Chemistry Real Life Examples These numbers are very important for telling us about the quantity of elements. The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. In chemistry, the mole is a unit used to talk about atoms. National chemistry week (ncw) mole day. It is similar to other units. Moles Chemistry Real Life Examples.
From www.youtube.com
How to Use the MOLE in Chemistry YouTube Moles Chemistry Real Life Examples Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions.. Moles Chemistry Real Life Examples.
From quizizz.com
Introduction to Moles Chemistry Quizizz Moles Chemistry Real Life Examples Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. This very large number is called avogadro’s number: These numbers are very important for telling us about the quantity of elements. 6.02214 x 1023, the number of units in a mole. The molar mass of a substance, in grams, is numerically. Moles Chemistry Real Life Examples.
From www.thoughtco.com
What Is a Mole in Chemistry? Moles Chemistry Real Life Examples The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. This very large number is called avogadro’s number: The mole is a key unit in chemistry. 6.02214 x 1023, the number of units in a mole. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the. Moles Chemistry Real Life Examples.
From dxolxludj.blob.core.windows.net
Titration Calculating Moles at Gary Mosley blog Moles Chemistry Real Life Examples These numbers are very important for telling us about the quantity of elements. In chemistry, the mole is a unit used to talk about atoms. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. The molar mass of a substance, in grams, is numerically equal to. Moles Chemistry Real Life Examples.
From www.showme.com
Mole calculations CHEM 1 Science, Chemistry, Moles ShowMe Moles Chemistry Real Life Examples The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. In chemistry, the mole is a unit used to talk about atoms. This very large number is called avogadro’s number: The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. It. Moles Chemistry Real Life Examples.
From www.slideserve.com
PPT Moles a counting unit in chemistry What is a mole? Why do we Moles Chemistry Real Life Examples In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. This very large number is called avogadro’s number: The mole is a key unit in chemistry. National chemistry week (ncw) mole day. These numbers are very important for telling us about the quantity of elements. 6.02214 x 1023, the. Moles Chemistry Real Life Examples.
From www.reddit.com
[Grade 11 Chemistry Calculating Moles with Uncertainties Using Moles Chemistry Real Life Examples These numbers are very important for telling us about the quantity of elements. 6.02214 x 1023, the number of units in a mole. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of. Moles Chemistry Real Life Examples.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube Moles Chemistry Real Life Examples Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. 6.02214 x 1023, the number of units in a mole. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. These numbers are very important for telling us about the. Moles Chemistry Real Life Examples.
From studylib.net
Chemistry Moles Moles Chemistry Real Life Examples It is similar to other units we use everyday. 6.02214 x 1023, the number of units in a mole. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as. Moles Chemistry Real Life Examples.
From www.showme.com
Basic mole conversions part 1 Science, Chemistry, Moles ShowMe Moles Chemistry Real Life Examples The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. 6.02214 x 1023, the number of units in a mole. In chemistry, the mole is a unit used to talk about atoms. The molar mass of a substance, in grams, is numerically equal to one atom's or. Moles Chemistry Real Life Examples.
From sciencenotes.org
What Is a Mole In Chemistry? Definition Moles Chemistry Real Life Examples Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. These numbers are very important for telling us about the quantity of elements. In chemistry, the mole is a unit used to talk about atoms. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding. Moles Chemistry Real Life Examples.
From www.pw.live
Number Of Moles Formula, Definition, Solved Examples Moles Chemistry Real Life Examples Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. 6.02214 x 1023, the number of units in a mole. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. It is similar to other units we use everyday.. Moles Chemistry Real Life Examples.
From ar.inspiredpencil.com
Chemistry Conversion Chart Moles To Grams Moles Chemistry Real Life Examples In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. National chemistry week (ncw) mole day. 6.02214 x 1023, the number of units in a mole. This very large number is called avogadro’s number: It is similar to other units we use everyday. Mole, standard unit (6.02214076 x 10^23). Moles Chemistry Real Life Examples.
From wou.edu
Chapter 6 Quantities in Chemical Reactions Chemistry Moles Chemistry Real Life Examples The mole is a key unit in chemistry. The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. It is similar to other units we use everyday. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. These numbers. Moles Chemistry Real Life Examples.
From intel-volley.blogspot.com
Learning is Fun! Chemistry Moles Moles Chemistry Real Life Examples In chemistry, the mole is a unit used to talk about atoms. This very large number is called avogadro’s number: Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very small entities such as atoms,. In chemistry, moles. Moles Chemistry Real Life Examples.
From www.pinterest.com
Chemistry and The o'jays on Pinterest Moles Chemistry Real Life Examples These numbers are very important for telling us about the quantity of elements. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a given sample. It is similar to other units we use everyday. The mole is a key unit in chemistry. Mole, standard unit (6.02214076 x 10^23) in chemistry for. Moles Chemistry Real Life Examples.
From www.pinterest.com
116 best Chemistry Moles, Stoichiometry, and Limiting Reagent images Moles Chemistry Real Life Examples It is similar to other units we use everyday. National chemistry week (ncw) mole day. The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. These numbers are very important for telling us about the quantity of elements. 6.02214 x 1023, the number of units in a mole. Mole, standard unit (6.02214076 x 10^23). Moles Chemistry Real Life Examples.
From telgurus.co.uk
How to calculate moles in chemistry? Chemistry questionnaire Moles Chemistry Real Life Examples The molar mass of a substance, in grams, is numerically equal to one atom's or molecule's. 6.02214 x 1023, the number of units in a mole. The mole is a key unit in chemistry. In chemistry, the mole is a unit used to talk about atoms. Mole, standard unit (6.02214076 x 10^23) in chemistry for measuring large quantities of very. Moles Chemistry Real Life Examples.
From materialmcgheeliteral.z21.web.core.windows.net
Converting Moles Worksheets Moles Chemistry Real Life Examples The mole, or “mol” is a unit of measurement in chemistry, used to designate a very large number of molecules, atoms, or particles. Contact your local acs outreach coordinator (ncw coordinator lookup or ccew coordinator lookup) or find an event (acs. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance in a. Moles Chemistry Real Life Examples.
From surfguppy.com
The Mole relationship to Carbon Surfguppy Chemistry made easy Moles Chemistry Real Life Examples In chemistry, the mole is a unit used to talk about atoms. This very large number is called avogadro’s number: The mole is a fundamental concept in the field of chemistry, serving as a cornerstone for understanding the quantitative aspects of chemical reactions. In chemistry, moles are a fundamental concept that is used to measure the amount of a substance. Moles Chemistry Real Life Examples.