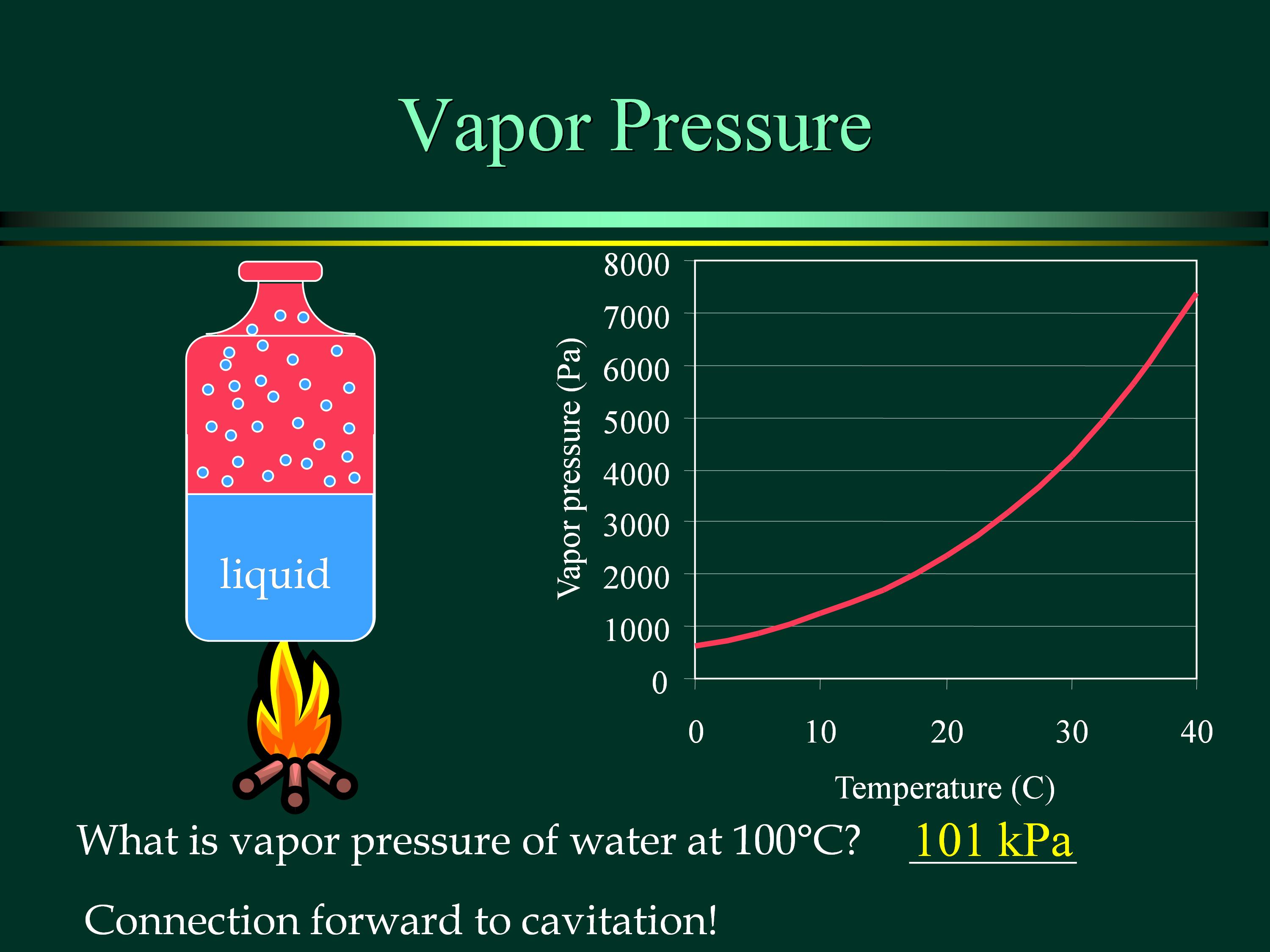
What Is Vapor Pressure Reduction . We observed that solute addition lowers the vapor pressure. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure mainly depends on. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic Boiling happens when the vapor pressure equals the atmospheric pressure. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. We can solve vapor pressure problems in either of two ways:
from ecoursesonline.iasri.res.in
The vapor pressure is the equilibrium partial pressure over a condensed phase. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We can solve vapor pressure problems in either of two ways: The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We observed that solute addition lowers the vapor pressure. Boiling happens when the vapor pressure equals the atmospheric pressure.
FM LESSON 3. PROPERTIES OF FLUID
What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. Boiling happens when the vapor pressure equals the atmospheric pressure. We observed that solute addition lowers the vapor pressure. We can solve vapor pressure problems in either of two ways: Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The vapor pressure mainly depends on. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 What Is Vapor Pressure Reduction We can solve vapor pressure problems in either of two ways: Boiling happens when the vapor pressure equals the atmospheric pressure. The vapor pressure mainly depends on. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. By using. What Is Vapor Pressure Reduction.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is Vapor Pressure Reduction We observed that solute addition lowers the vapor pressure. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The. What Is Vapor Pressure Reduction.
From www.pipingengineer.org
Centrifugal Pump Terminology The Piping Engineering World What Is Vapor Pressure Reduction The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. We can solve vapor pressure problems in either of two ways: We observed that solute addition lowers the vapor pressure. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We need two pieces of information to calculate the. What Is Vapor Pressure Reduction.
From chem.libretexts.org
13.6 Vapor Pressures of Solutions Chemistry LibreTexts What Is Vapor Pressure Reduction The vapor pressure mainly depends on. We can solve vapor pressure problems in either of two ways: The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The vapor pressure. What Is Vapor Pressure Reduction.
From socratic.org
What is the relation between critical temperature and boiling point or What Is Vapor Pressure Reduction The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The vapor pressure of the sodium. What Is Vapor Pressure Reduction.
From webgiasi.vn
Which molecules have higher (or lower) vapor pressure vapor pressure What Is Vapor Pressure Reduction The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. We observed that solute addition lowers the vapor pressure. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We need. What Is Vapor Pressure Reduction.
From www.youtube.com
What is meant by Vapour Pressure (in Urdu / Hindi) How is vapor What Is Vapor Pressure Reduction The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The vapor pressure mainly depends on. We observed that solute addition lowers the vapor pressure. We can solve vapor pressure problems in either of two ways: We need two pieces of information to calculate the reduction of the vapor pressure. What Is Vapor Pressure Reduction.
From worksheetdbwounds.z13.web.core.windows.net
Vapor Pressure And Boiling Worksheet What Is Vapor Pressure Reduction We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. The vapor pressure is the equilibrium partial pressure over a condensed phase. We can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The concentration. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Colligative Properties PowerPoint Presentation, free download What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We observed that solute addition lowers the vapor pressure. The. What Is Vapor Pressure Reduction.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The vapor pressure mainly depends on. The vapor. What Is Vapor Pressure Reduction.
From ecoursesonline.iasri.res.in
FM LESSON 3. PROPERTIES OF FLUID What Is Vapor Pressure Reduction We observed that solute addition lowers the vapor pressure. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. The vapor pressure mainly depends on. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. By using. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Colligative properties PowerPoint Presentation, free download What Is Vapor Pressure Reduction The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We can solve vapor pressure problems in either of two ways: Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The vapor pressure mainly depends on. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a.. What Is Vapor Pressure Reduction.
From scienceinfo.com
Lowering of Vapor Pressure A Colligative property What Is Vapor Pressure Reduction The vapor pressure mainly depends on. We can solve vapor pressure problems in either of two ways: The vapor pressure is the equilibrium partial pressure over a condensed phase. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions.. What Is Vapor Pressure Reduction.
From www.youtube.com
Colligative properties Vapor pressure lowering YouTube What Is Vapor Pressure Reduction We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. The vapor pressure is the equilibrium partial pressure over a condensed phase. We can solve vapor pressure problems in either of two ways: The vapor pressure mainly depends on. We observed that solute addition lowers the vapor. What Is Vapor Pressure Reduction.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID2755893 What Is Vapor Pressure Reduction Boiling happens when the vapor pressure equals the atmospheric pressure. We observed that solute addition lowers the vapor pressure. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID7038735 What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The vapor pressure mainly depends on. We can solve vapor pressure problems in either of two ways: We. What Is Vapor Pressure Reduction.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is Vapor Pressure Reduction The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic By using equation \ref{13.6.1} to calculate the actual. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Vapor Pressure Lowering PowerPoint Presentation, free download What Is Vapor Pressure Reduction The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Boiling happens when the vapor pressure equals the atmospheric pressure. We observed that solute addition lowers the vapor pressure. The vapor pressure of the sodium chloride solution will. What Is Vapor Pressure Reduction.
From slideplayer.com
Drill 4/11/08 What two factors determine if a substance is in the What Is Vapor Pressure Reduction Boiling happens when the vapor pressure equals the atmospheric pressure. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The vapor pressure is the equilibrium partial pressure over a condensed phase. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 What Is Vapor Pressure Reduction We can solve vapor pressure problems in either of two ways: The vapor pressure mainly depends on. Boiling happens when the vapor pressure equals the atmospheric pressure. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The vapor pressure is the equilibrium partial pressure over a condensed phase. The concentration of. What Is Vapor Pressure Reduction.
From amariqoleon.blogspot.com
What is Vapor Pressure AmariqoLeon What Is Vapor Pressure Reduction The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. Vapour pressure reduction, boiling point elevation, freezing. What Is Vapor Pressure Reduction.
From engineerexcel.com
Vapor Pressure of Water Explained EngineerExcel What Is Vapor Pressure Reduction We can solve vapor pressure problems in either of two ways: The vapor pressure mainly depends on. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We observed that solute addition lowers the vapor pressure. The concentration of solute molecules or ions, but not the identity of the solute,. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID1980276 What Is Vapor Pressure Reduction The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. We can solve vapor pressure problems in either of two ways: The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. By using equation \ref{13.6.1} to calculate the actual vapor pressure above. What Is Vapor Pressure Reduction.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Reduction We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. We can solve vapor pressure problems in either of two ways: By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a solvent in a solution is always lower than the. What Is Vapor Pressure Reduction.
From inspiritvr.com
Colligative Properties Study Guide Inspirit What Is Vapor Pressure Reduction The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic By using equation \ref{13.6.1} to calculate. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT 154 Colligative Properties PowerPoint Presentation, free What Is Vapor Pressure Reduction Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We can solve vapor pressure problems in either of two ways: The vapor pressure mainly depends on. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. By using equation \ref{13.6.1} to calculate the actual vapor. What Is Vapor Pressure Reduction.
From study.com
Vapor Pressure Definition, Equation & Examples Video & Lesson What Is Vapor Pressure Reduction The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. Boiling happens when the vapor pressure equals the atmospheric pressure. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We observed that solute addition lowers the vapor pressure. The vapor pressure is the equilibrium partial pressure over a. What Is Vapor Pressure Reduction.
From slideplayer.com
Ask a chemist, they always have ppt download What Is Vapor Pressure Reduction The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We observed that solute addition lowers the vapor pressure. The vapor pressure is the equilibrium partial pressure over a condensed phase. The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. We. What Is Vapor Pressure Reduction.
From general.chemistrysteps.com
Vapor Pressure Lowering Chemistry Steps What Is Vapor Pressure Reduction By using equation \ref{13.6.1} to calculate the actual vapor pressure above a. The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. The vapor pressure is the equilibrium partial pressure over a condensed phase. Boiling happens when the vapor pressure equals the atmospheric pressure. The vapor pressure of the sodium. What Is Vapor Pressure Reduction.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is Vapor Pressure Reduction We observed that solute addition lowers the vapor pressure. We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic The vapor pressure mainly depends on. The vapor pressure of the sodium chloride solution will be lowered. What Is Vapor Pressure Reduction.
From chemyam.blogspot.com
COLLIGATIVE PROPERTIES (Relative lowering of vapour pressure) What Is Vapor Pressure Reduction Boiling happens when the vapor pressure equals the atmospheric pressure. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We need two pieces of information to calculate the reduction of the vapor pressure of the solvent in a solution containing a nonvolatile. The vapor pressure mainly depends on. We observed that solute addition lowers the vapor pressure. The. What Is Vapor Pressure Reduction.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download What Is Vapor Pressure Reduction The vapor pressure of a solvent in a solution is always lower than the vapor pressure of the pure solvent. We can solve vapor pressure problems in either of two ways: The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. By using equation \ref{13.6.1} to calculate the actual vapor. What Is Vapor Pressure Reduction.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit What Is Vapor Pressure Reduction We can solve vapor pressure problems in either of two ways: The vapor pressure of the sodium chloride solution will be lowered twice the amount of the glucose solution. The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. We need two pieces of information to calculate the reduction of. What Is Vapor Pressure Reduction.
From saylordotorg.github.io
Vapor Pressure What Is Vapor Pressure Reduction The vapor pressure is the equilibrium partial pressure over a condensed phase. Vapour pressure reduction, boiling point elevation, freezing point depression, and osmotic We can solve vapor pressure problems in either of two ways: The concentration of solute molecules or ions, but not the identity of the solute, determines the colligative characteristics of solutions. By using equation \ref{13.6.1} to calculate. What Is Vapor Pressure Reduction.