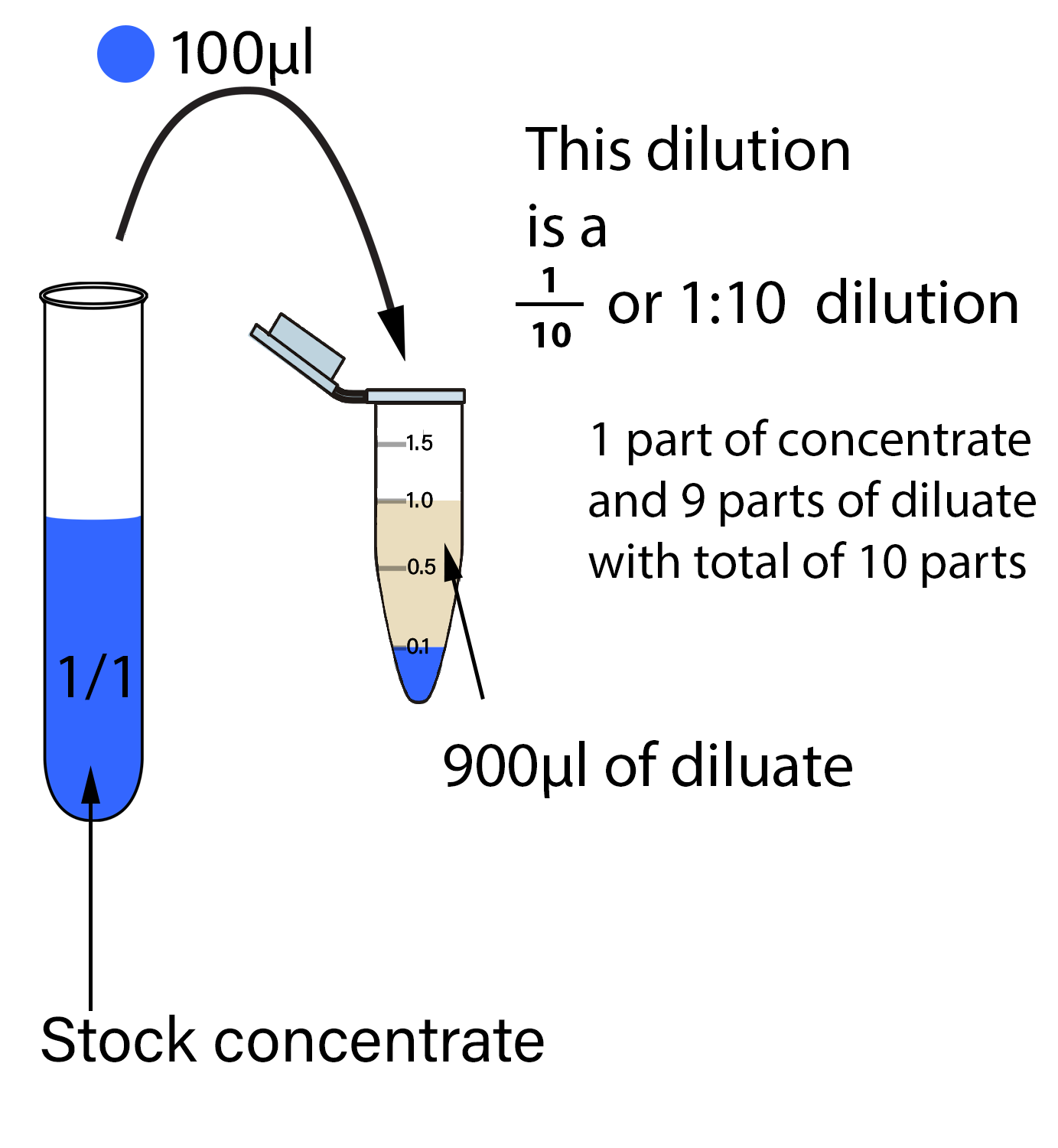
In Dilution Volume . imagine we have a salt water solution with a certain concentration. Using the dilution equation, we write: M₁v₁ = m₂v₂ where m₁. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. That means we have a certain amount of salt (a certain mass or a. we can relate the concentrations and volumes before and after a dilution using the following equation: state whether the concentration of a solution is directly or indirectly proportional to its volume.
from exocdvozc.blob.core.windows.net
we can relate the concentrations and volumes before and after a dilution using the following equation: imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt (a certain mass or a. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. Using the dilution equation, we write: dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. M₁v₁ = m₂v₂ where m₁.
Dilute 30 Volume To 10 at Michelle Isakson blog
In Dilution Volume Using the dilution equation, we write: Using the dilution equation, we write: we can relate the concentrations and volumes before and after a dilution using the following equation: the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. imagine we have a salt water solution with a certain concentration. M₁v₁ = m₂v₂ where m₁. That means we have a certain amount of salt (a certain mass or a. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state whether the concentration of a solution is directly or indirectly proportional to its volume.
From exookhlfi.blob.core.windows.net
Dilution Factor Calculation In Hplc at John Comer blog In Dilution Volume the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. we can relate the concentrations and volumes before and after a dilution using the following equation: Using the dilution equation, we write: state whether the concentration of a solution is directly or indirectly proportional to its volume. M₁v₁. In Dilution Volume.
From www.youtube.com
Dilution Chart.Helpful video. Understand how to prepare dilutions in Lab.Understand & clear In Dilution Volume Using the dilution equation, we write: That means we have a certain amount of salt (a certain mass or a. we can relate the concentrations and volumes before and after a dilution using the following equation: the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether. In Dilution Volume.
From www.researchgate.net
Effect of change in dilution volume and pH on mean droplet size (Mean ±... Download Scientific In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution is directly or indirectly proportional to its volume. imagine we have a salt water solution with a certain concentration. . In Dilution Volume.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Example 2 YouTube In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. imagine we have a salt water solution with a certain concentration. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a. In Dilution Volume.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution. In Dilution Volume.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog In Dilution Volume That means we have a certain amount of salt (a certain mass or a. imagine we have a salt water solution with a certain concentration. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. Using the. In Dilution Volume.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and chemistry YouTube In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. M₁v₁ = m₂v₂ where m₁. Using the dilution equation, we write: state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. . In Dilution Volume.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog In Dilution Volume the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. That means we have a certain amount of salt (a certain mass or a. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. we can relate the. In Dilution Volume.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog In Dilution Volume imagine we have a salt water solution with a certain concentration. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. That means we have a certain amount of salt (a certain mass or a. state whether the concentration of a solution is directly or indirectly proportional to its volume. we can relate the concentrations and. In Dilution Volume.
From slidetodoc.com
Dilution of Solutions 1 Volume to volume dilutions In Dilution Volume the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. we can relate the concentrations and volumes before and after a dilution using the following equation: dilution is the process of decreasing the concentration of a solute. In Dilution Volume.
From www.researchgate.net
Dilution process of the sample. V a (ml) is aliquot volume and N,... Download Scientific Diagram In Dilution Volume imagine we have a salt water solution with a certain concentration. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. state whether the concentration of a solution is directly or indirectly proportional to its volume. M₁v₁ = m₂v₂ where m₁. the dilution ratio calculator tells. In Dilution Volume.
From exosmtljy.blob.core.windows.net
Dilution Cell Density at Bryan Owen blog In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt (a certain mass or a. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. state. In Dilution Volume.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog In Dilution Volume imagine we have a salt water solution with a certain concentration. That means we have a certain amount of salt (a certain mass or a. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. dilution is the process of decreasing the concentration of a solute in a. In Dilution Volume.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog In Dilution Volume state whether the concentration of a solution is directly or indirectly proportional to its volume. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. we can relate the concentrations and volumes before and after a dilution. In Dilution Volume.
From www.answersarena.com
[Solved] Dilution diagrams can be very helpful in organiz In Dilution Volume the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Using the dilution equation, we write: M₁v₁ = m₂v₂ where m₁. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by. In Dilution Volume.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. M₁v₁ = m₂v₂ where m₁. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. That means we have a certain amount of salt (a certain mass or a. Using the dilution equation, we write: state whether. In Dilution Volume.
From www.slideshare.net
Molarity and dilution In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. That means we have a certain amount of salt (a certain mass or a. imagine we have a salt water solution with a certain concentration. Using the dilution. In Dilution Volume.
From labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory, and Dilution Formulas In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. state whether the concentration of a solution is directly or indirectly proportional to its volume. imagine we have a salt water solution with a certain concentration. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100.. In Dilution Volume.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. state whether the concentration of a solution is directly or indirectly proportional to its volume. M₁v₁ = m₂v₂ where m₁. Using the dilution equation, we write: the dilution ratio calculator tells you how much solute and solvent. In Dilution Volume.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. That means we have a certain amount of salt (a certain mass or a. M₁v₁ = m₂v₂ where m₁. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process of decreasing the concentration of a solute in a. In Dilution Volume.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution is directly or indirectly proportional to its volume. imagine we have a salt water solution with a certain concentration. That. In Dilution Volume.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. Using the dilution equation, we write: the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution is directly or indirectly proportional to its volume. we can relate the concentrations and. In Dilution Volume.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. we can relate the concentrations and volumes before and after a dilution using the following equation: Using the. In Dilution Volume.
From exoaaybok.blob.core.windows.net
Dilution Method Microbiology at Harry Hausman blog In Dilution Volume M₁v₁ = m₂v₂ where m₁. imagine we have a salt water solution with a certain concentration. Using the dilution equation, we write: (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. That means we have a certain. In Dilution Volume.
From dxohqxlgl.blob.core.windows.net
Dilution 1 Volume at Jeremiah Gonzalez blog In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. imagine we have a salt water solution with a certain concentration. M₁v₁ = m₂v₂ where m₁. the dilution ratio calculator tells you how much solute and. In Dilution Volume.
From dxosnjafu.blob.core.windows.net
Dilution Calculation Videos at Iva Fugate blog In Dilution Volume imagine we have a salt water solution with a certain concentration. Using the dilution equation, we write: the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800. In Dilution Volume.
From twinklsecondary.blog
Products of a Dilution Series A Level Biology Revision In Dilution Volume dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. That means we have a certain amount of salt (a certain mass or a. state whether the concentration of a solution is directly or indirectly proportional to its volume. (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x. In Dilution Volume.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY In Dilution Volume Using the dilution equation, we write: we can relate the concentrations and volumes before and after a dilution using the following equation: imagine we have a salt water solution with a certain concentration. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. M₁v₁ = m₂v₂ where. In Dilution Volume.
From exocdvozc.blob.core.windows.net
Dilute 30 Volume To 10 at Michelle Isakson blog In Dilution Volume imagine we have a salt water solution with a certain concentration. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. we can relate the concentrations and volumes before and after a dilution using the following equation: That means we have a certain amount of salt (a. In Dilution Volume.
From www.chegg.com
Solved 2. Bradford Assay. The total dilution volume is 100 In Dilution Volume M₁v₁ = m₂v₂ where m₁. That means we have a certain amount of salt (a certain mass or a. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. state whether the concentration of a solution is directly or indirectly proportional to its volume. dilution is the process. In Dilution Volume.
From www.chegg.com
Solved The most common dilutions are 1/10, 1/1000 and In Dilution Volume state whether the concentration of a solution is directly or indirectly proportional to its volume. Using the dilution equation, we write: imagine we have a salt water solution with a certain concentration. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the dilution ratio calculator. In Dilution Volume.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog In Dilution Volume we can relate the concentrations and volumes before and after a dilution using the following equation: Using the dilution equation, we write: dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. the dilution ratio calculator tells you how much solute and solvent you need to get. In Dilution Volume.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog In Dilution Volume we can relate the concentrations and volumes before and after a dilution using the following equation: M₁v₁ = m₂v₂ where m₁. the dilution ratio calculator tells you how much solute and solvent you need to get the desired dilution ratio. imagine we have a salt water solution with a certain concentration. (1.50 mol/l) (53.4 ml) = (0.800. In Dilution Volume.
From dxobhpmpa.blob.core.windows.net
Dilution Mass Per Volume at Thomas Bowling blog In Dilution Volume M₁v₁ = m₂v₂ where m₁. dilution is the process of decreasing the concentration of a solute in a solution, usually simply by mixing with more. state whether the concentration of a solution is directly or indirectly proportional to its volume. imagine we have a salt water solution with a certain concentration. Using the dilution equation, we write:. In Dilution Volume.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution factor in Serial In Dilution Volume (1.50 mol/l) (53.4 ml) = (0.800 mol/l) (x) x = 100. we can relate the concentrations and volumes before and after a dilution using the following equation: Using the dilution equation, we write: M₁v₁ = m₂v₂ where m₁. That means we have a certain amount of salt (a certain mass or a. the dilution ratio calculator tells you. In Dilution Volume.