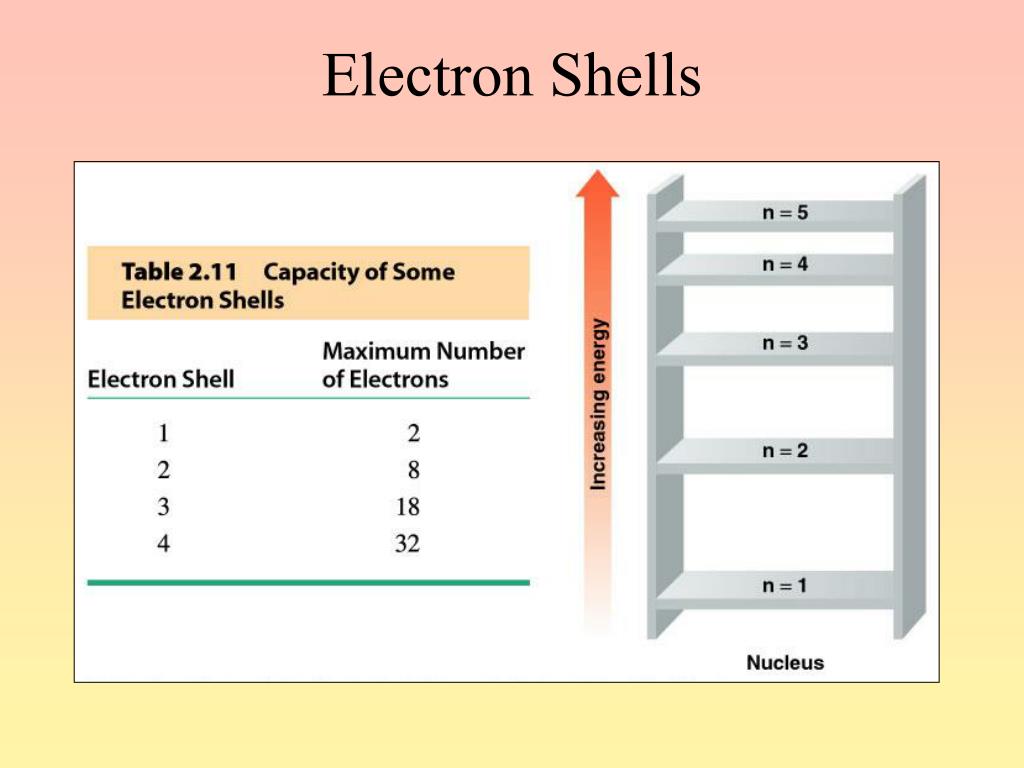
Which Electron Shell Has The Highest Energy Level . in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. The next atom after neon, sodium , has. shells, subshells, and orbitals. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels.
from www.slideserve.com
electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. The next atom after neon, sodium , has.
PPT Electron Energy Levels PowerPoint Presentation, free download ID1377451
Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. The next atom after neon, sodium , has. shells, subshells, and orbitals. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
From stock.adobe.com
chart of electron configuration with each energy level for element in chemistry. Stock Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. electrons can either jump to a higher energy level by. Which Electron Shell Has The Highest Energy Level.
From www.goodscience.com.au
Electron Configuration (Elements 120) Good Science Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. so a large amount. Which Electron Shell Has The Highest Energy Level.
From www.slideserve.com
PPT Electron Energy Levels PowerPoint Presentation, free download ID1377451 Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. The next atom after neon, sodium , has. electrons can either jump to a. Which Electron Shell Has The Highest Energy Level.
From newtondesk.com
Periodic Elements Electron Shells, SubShells, and Orbitals Chemistry Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. shells, subshells, and orbitals. The next atom after neon, sodium , has. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes. Which Electron Shell Has The Highest Energy Level.
From www.periodictableprintable.com
Periodic Table With Electron Structure Of Energy Levels 2023 Periodic Table Printable Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. shells, subshells, and orbitals. The next atom after neon, sodium , has. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or. Which Electron Shell Has The Highest Energy Level.
From commons.wikimedia.org
FilePeriodic table of elements showing electron shells.png Which Electron Shell Has The Highest Energy Level The next atom after neon, sodium , has. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. so a large amount of energy is required to liberate an electron from an inner most shell rather than. Which Electron Shell Has The Highest Energy Level.
From www.slideserve.com
PPT Electrons and the Periodic Table PowerPoint Presentation, free download ID6502309 Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. The next atom after neon, sodium , has. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. this shell has. Which Electron Shell Has The Highest Energy Level.
From hubpages.com
Atoms and Atomic Structure Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. this shell has spaces for eight. Which Electron Shell Has The Highest Energy Level.
From pijaeducation.com
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. The next atom after neon, sodium , has. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. shells, subshells,. Which Electron Shell Has The Highest Energy Level.
From www.thoughtco.com
Electron Configuration Chart Which Electron Shell Has The Highest Energy Level shells, subshells, and orbitals. The next atom after neon, sodium , has. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy. Which Electron Shell Has The Highest Energy Level.
From pediabay.com
Periodic Table & Energy Levels (Electrons per shell) Pediabay Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. shells, subshells, and orbitals. electrons can either jump to a higher energy level. Which Electron Shell Has The Highest Energy Level.
From lessondbchandelles.z21.web.core.windows.net
Bohr's Atomic Model Notes Which Electron Shell Has The Highest Energy Level The next atom after neon, sodium , has. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. shells, subshells, and. Which Electron Shell Has The Highest Energy Level.
From www.slideserve.com
PPT Electrons and the Periodic Table PowerPoint Presentation, free download ID6502309 Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. in this section we will discuss the energy level. Which Electron Shell Has The Highest Energy Level.
From alevelchemistry.co.uk
Electron Configurations Orbitals, Energy Levels and Ionisation Energy Trends ALevel Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. The next atom after neon, sodium , has. so a large amount of energy is required to liberate an electron from an inner most shell rather than. Which Electron Shell Has The Highest Energy Level.
From sciencing.com
Energy Level Definition, Equation (w/ Diagrams) Sciencing Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. so a large amount of energy is required to. Which Electron Shell Has The Highest Energy Level.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica Which Electron Shell Has The Highest Energy Level this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. shells, subshells, and orbitals. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. electrons can either jump to a higher energy level. Which Electron Shell Has The Highest Energy Level.
From newtondesk.com
Electron Configuration of Elements Chemistry Periodic Table Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. shells, subshells, and orbitals. this shell has spaces for eight. Which Electron Shell Has The Highest Energy Level.
From evulpo.com
Electron energy levels and ionisation Physics Explanation & Exercises evulpo Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing,. Which Electron Shell Has The Highest Energy Level.
From www.online-sciences.com
Atomic structure of matter, Energy levels, Electronic distribution and chemical activity Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. in this section we will discuss the energy level of the electron of a. Which Electron Shell Has The Highest Energy Level.
From cronodon.com
Introduction to Atoms Which Electron Shell Has The Highest Energy Level The next atom after neon, sodium , has. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. shells, subshells, and orbitals. in. Which Electron Shell Has The Highest Energy Level.
From acemichael888.weebly.com
Electron Arrangement in Atoms Elements and the Periodic Table Which Electron Shell Has The Highest Energy Level The next atom after neon, sodium , has. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes. Which Electron Shell Has The Highest Energy Level.
From www.animalia-life.club
Electron Shell Diagram Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. electrons can either jump to a higher energy level by. Which Electron Shell Has The Highest Energy Level.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. The next atom after neon, sodium , has. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. in this section we will discuss. Which Electron Shell Has The Highest Energy Level.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Image License Carlson Stock Art Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. The next atom after neon, sodium. Which Electron Shell Has The Highest Energy Level.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho Which Electron Shell Has The Highest Energy Level shells, subshells, and orbitals. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. so a large amount of. Which Electron Shell Has The Highest Energy Level.
From www.stonecoldhands.com
Electron Shells and Orbitals Stone Cold Chemistry Talk Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. shells, subshells, and orbitals. The next atom after neon, sodium , has. electrons. Which Electron Shell Has The Highest Energy Level.
From www.chemistrystudent.com
Electron Orbitals (ALevel) ChemistryStudent Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. The next atom after neon, sodium ,. Which Electron Shell Has The Highest Energy Level.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry Which Electron Shell Has The Highest Energy Level shells, subshells, and orbitals. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. electrons can either jump to a higher energy level. Which Electron Shell Has The Highest Energy Level.
From www.slideserve.com
PPT Catalyst PowerPoint Presentation, free download ID4302021 Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. this. Which Electron Shell Has The Highest Energy Level.
From www.nagwa.com
Question Video Ranking Three Electrons from Lowest to Highest Energy Given the Quantum Numbers Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. shells, subshells, and orbitals. electrons can either jump to a. Which Electron Shell Has The Highest Energy Level.
From stock.adobe.com
illustration of chemistry, Electron shell diagram,atomic shell is a graphical representation of Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. The next atom after neon, sodium , has. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell. Which Electron Shell Has The Highest Energy Level.
From sciencenotes.org
Electron Shell Diagrams of the 118 Elements Which Electron Shell Has The Highest Energy Level electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. The next atom after neon, sodium , has. in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes. Which Electron Shell Has The Highest Energy Level.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube Which Electron Shell Has The Highest Energy Level The next atom after neon, sodium , has. so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. shells, subshells, and orbitals. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or. Which Electron Shell Has The Highest Energy Level.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Which Electron Shell Has The Highest Energy Level so a large amount of energy is required to liberate an electron from an inner most shell rather than an electron. this shell has spaces for eight electrons, so that it takes an atom with 10 electrons to fill the first two levels. in this section we will discuss the energy level of the electron of a. Which Electron Shell Has The Highest Energy Level.
From slidetodoc.com
Electrons Sections 2 5 2 7 Electrons energy Which Electron Shell Has The Highest Energy Level in this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. electrons can either jump to a higher energy level by absorbing, or gaining energy, or drop to a lower energy level by emitting, or losing energy. shells, subshells, and orbitals. so. Which Electron Shell Has The Highest Energy Level.