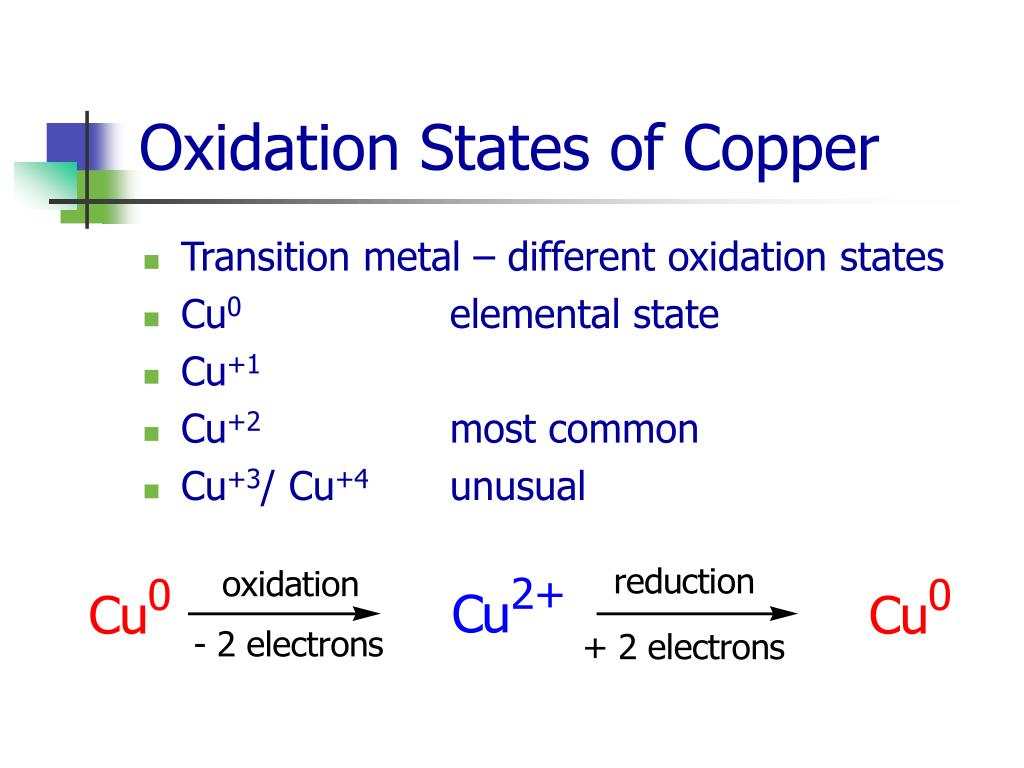
Copper Oxidation Number . This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Find the oxidation numbers of the elements in a chemical formula using this online tool. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. The sum of the oxidation states within a compound or ion must equal the overall charge. The oxidation number of metallic copper is zero. Uncombined elements have an oxidation state of 0. In its compounds the most common oxidation number of cu is +2. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Learn what an oxidation state is and how to calculate.
from www.slideserve.com
The sum of the oxidation states within a compound or ion must equal the overall charge. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Learn what an oxidation state is and how to calculate. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Uncombined elements have an oxidation state of 0. Find the oxidation numbers of the elements in a chemical formula using this online tool. In its compounds the most common oxidation number of cu is +2. The oxidation number of metallic copper is zero. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient.
PPT Experiment 3 PowerPoint Presentation, free download ID243127
Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Uncombined elements have an oxidation state of 0. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. The oxidation number of metallic copper is zero. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. In its compounds the most common oxidation number of cu is +2. The sum of the oxidation states within a compound or ion must equal the overall charge. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn what an oxidation state is and how to calculate.
From www.doubtnut.com
The coordination number and oxidation state of copper in 'F' is Copper Oxidation Number The sum of the oxidation states within a compound or ion must equal the overall charge. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. The oxidation number of metallic copper is zero. Learn what. Copper Oxidation Number.
From www.chegg.com
Solved 26. In the oxidation of copper, Model The Oxidation Copper Oxidation Number Learn what an oxidation state is and how to calculate. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Uncombined elements have an oxidation state of 0. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. The oxidation number of metallic copper is zero. The sum of the. Copper Oxidation Number.
From www.youtube.com
Balance the chemical equation by the oxidation number method. (i) `CuO Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. Find the oxidation numbers of the elements in a chemical formula using this online tool. The oxidation number of metallic copper is zero. Learn what an oxidation state is and how to calculate.. Copper Oxidation Number.
From quizlet.com
Fe, Sn, Pb, and Cu Oxidation Numbers Diagram Quizlet Copper Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Keeping the atomic orbitals when assigning oxidation numbers in mind. Copper Oxidation Number.
From www.compoundchem.com
Compound Interest The Periodic Table of Oxidation States Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient.. Copper Oxidation Number.
From www.researchgate.net
The different copper oxidation states, average copper oxidation state Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. Uncombined elements have an oxidation state of 0. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states (oxidation numbers) are and. Copper Oxidation Number.
From moms-hub.blogspot.com
Moms Hub how to calculate oxidation number in a reaction Copper Oxidation Number Learn what an oxidation state is and how to calculate. Uncombined elements have an oxidation state of 0. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Find the oxidation numbers of the elements in a chemical formula using this online tool. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in. Copper Oxidation Number.
From www.chegg.com
Solved Give the oxidation number for each element in each Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. In its compounds the most common oxidation number of cu is +2. The sum of the oxidation states within a compound or ion must equal. Copper Oxidation Number.
From blog.thepipingmart.com
Copper Oxidation States and Colors Copper Oxidation Number Uncombined elements have an oxidation state of 0. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose. Copper Oxidation Number.
From chem.libretexts.org
Oxidation States of Transition Metals Chemistry LibreTexts Copper Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. In its compounds. Copper Oxidation Number.
From www.chegg.com
Solved Classify the copper compounds by the copper oxidation Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Learn what an oxidation state is and how to calculate. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them.. Copper Oxidation Number.
From resource.studiaacademy.com
Chemical Formulae, Equations and Calculations Studia Academy Resources Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. The oxidation number of metallic copper is zero. The sum of the oxidation states within a compound or ion must equal the overall charge. Find the oxidation numbers of the elements in a. Copper Oxidation Number.
From www.periodic-table.org
Copper Electron Configuration and Oxidation States Cu Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. In its compounds the. Copper Oxidation Number.
From www.youtube.com
balance by oxidation number method Cu + NO3 to give NO2 + Cu2+ Copper Oxidation Number Learn what an oxidation state is and how to calculate. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Oxidation number of copper can be +1 or +2, depending on its chemical structure. In its compounds the most common oxidation number of cu is +2. Find the oxidation numbers of. Copper Oxidation Number.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples Copper Oxidation Number In its compounds the most common oxidation number of cu is +2. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Find the oxidation numbers of the elements in a chemical formula using this online tool. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. This page. Copper Oxidation Number.
From www.chemistrystudent.com
Electrochemical Cells (ALevel) ChemistryStudent Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. In its compounds the most common oxidation number of cu is +2. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states. Copper Oxidation Number.
From www.toppr.com
(i) Balance the following equations by oxidation number method (1) Cu Copper Oxidation Number The oxidation number of metallic copper is zero. Uncombined elements have an oxidation state of 0. The sum of the oxidation states within a compound or ion must equal the overall charge. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. Copper’s. Copper Oxidation Number.
From www.researchgate.net
Oxidation states of Cu, Sn, and S in various binary and ternary Copper Oxidation Number The sum of the oxidation states within a compound or ion must equal the overall charge. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn what an oxidation state is and how to calculate. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Uncombined elements have an oxidation. Copper Oxidation Number.
From www.linstitute.net
AQA A Level Chemistry复习笔记6.2.5 Variable Oxidation States翰林国际教育 Copper Oxidation Number The oxidation number of metallic copper is zero. Uncombined elements have an oxidation state of 0. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. The sum of the oxidation states within a compound or ion must equal the overall charge. In its compounds the most common oxidation number of cu is +2. Keeping. Copper Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for Cu in CuO Copper (II) oxide Copper Oxidation Number In its compounds the most common oxidation number of cu is +2. Oxidation number of copper can be +1 or +2, depending on its chemical structure. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. The oxidation number of metallic copper is zero. Keeping the atomic orbitals when assigning oxidation. Copper Oxidation Number.
From www.chegg.com
Solved The compound Na4ICu(NO2)6l is Determine Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Learn what an oxidation state is and how to calculate. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2.. Copper Oxidation Number.
From www.slideserve.com
PPT Oxidation and Reduction Reactions PowerPoint Presentation, free Copper Oxidation Number In its compounds the most common oxidation number of cu is +2. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Learn what an oxidation state is and how to calculate. Find the oxidation numbers of the elements in a chemical formula using this online tool. Copper’s +1 oxidation state. Copper Oxidation Number.
From pubs.rsc.org
The oxidation of copper catalysts during ethylene epoxidation Copper Oxidation Number The sum of the oxidation states within a compound or ion must equal the overall charge. In its compounds the most common oxidation number of cu is +2. The oxidation number of metallic copper is zero. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Learn what an oxidation state is and how to calculate.. Copper Oxidation Number.
From www.slideserve.com
PPT Experiment 3 PowerPoint Presentation, free download ID243127 Copper Oxidation Number Oxidation number of copper can be +1 or +2, depending on its chemical structure. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. The sum of the oxidation states within a compound or ion must equal the overall charge. The oxidation number. Copper Oxidation Number.
From www.slideserve.com
PPT Balancing equations using oxidation numbers PowerPoint Copper Oxidation Number Learn what an oxidation state is and how to calculate. The oxidation number of metallic copper is zero. This page explains what oxidation states (oxidation numbers) are and how to calculate them and make use of them. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Uncombined elements have an oxidation state of 0. Keeping. Copper Oxidation Number.
From www.slideserve.com
PPT Chapter 6 OxidationReduction Reactions PowerPoint Presentation Copper Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page explains what oxidation states (oxidation numbers) are and how to calculate them. Copper Oxidation Number.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Copper Oxidation Number Oxidation number of copper can be +1 or +2, depending on its chemical structure. Learn what an oxidation state is and how to calculate. Find the oxidation numbers of the elements in a chemical formula using this online tool. The sum of the oxidation states within a compound or ion must equal the overall charge. Copper’s +1 oxidation state is. Copper Oxidation Number.
From brainly.in
What is oxidation number of N in Cu(NO3)2 ? Plz show me how did you Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. The sum of the oxidation states within a compound or ion must equal the overall charge. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Learn. Copper Oxidation Number.
From www.vrogue.co
Ppt Oxidation Reduction Powerpoint Presentation Free vrogue.co Copper Oxidation Number Uncombined elements have an oxidation state of 0. Find the oxidation numbers of the elements in a chemical formula using this online tool. Learn what an oxidation state is and how to calculate. Oxidation number of copper can be +1 or +2, depending on its chemical structure. Copper’s +1 oxidation state is common in compounds such as cucl and cuo,. Copper Oxidation Number.
From www.chegg.com
Solved What is the oxidation number for copper in CuSO4? Copper Oxidation Number Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Oxidation number of copper can be +1 or +2, depending on its chemical structure. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. The oxidation number of metallic copper is zero. Learn what an oxidation state is and. Copper Oxidation Number.
From study.com
Oxidation State of Transition Metals Definition & Overview Lesson Copper Oxidation Number Learn what an oxidation state is and how to calculate. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. The oxidation number of metallic copper is zero. Uncombined elements have an oxidation state of 0. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a. Copper Oxidation Number.
From www.nagwa.com
Question Video Identifying Which Species Are Oxidized and Reduced by Copper Oxidation Number This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. This page. Copper Oxidation Number.
From www.slideserve.com
PPT Reduction Oxidation PowerPoint Presentation, free download ID Copper Oxidation Number Keeping the atomic orbitals when assigning oxidation numbers in mind helps in recognizing that transition metals pose a special case, but not an exception to this convenient. The sum of the oxidation states within a compound or ion must equal the overall charge. Oxidation number of copper can be +1 or +2, depending on its chemical structure. This page explains. Copper Oxidation Number.
From www.youtube.com
How to find the Oxidation Number for Cu in CuBr2 YouTube Copper Oxidation Number Learn what an oxidation state is and how to calculate. The oxidation number of metallic copper is zero. Uncombined elements have an oxidation state of 0. Find the oxidation numbers of the elements in a chemical formula using this online tool. This page explains what oxidation states (oxidation numbers) are and how to calculate and use them. Copper’s +1 oxidation. Copper Oxidation Number.
From worksheetlistsog.z21.web.core.windows.net
How To Do Oxidation Numbers In Chemistry Copper Oxidation Number Find the oxidation numbers of the elements in a chemical formula using this online tool. Copper’s +1 oxidation state is common in compounds such as cucl and cuo, while +2. Uncombined elements have an oxidation state of 0. Oxidation number of copper can be +1 or +2, depending on its chemical structure. The sum of the oxidation states within a. Copper Oxidation Number.