Iron Electron Donor . Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing in rock formations.
from donate-faqs.com
Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing in rock formations. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron.
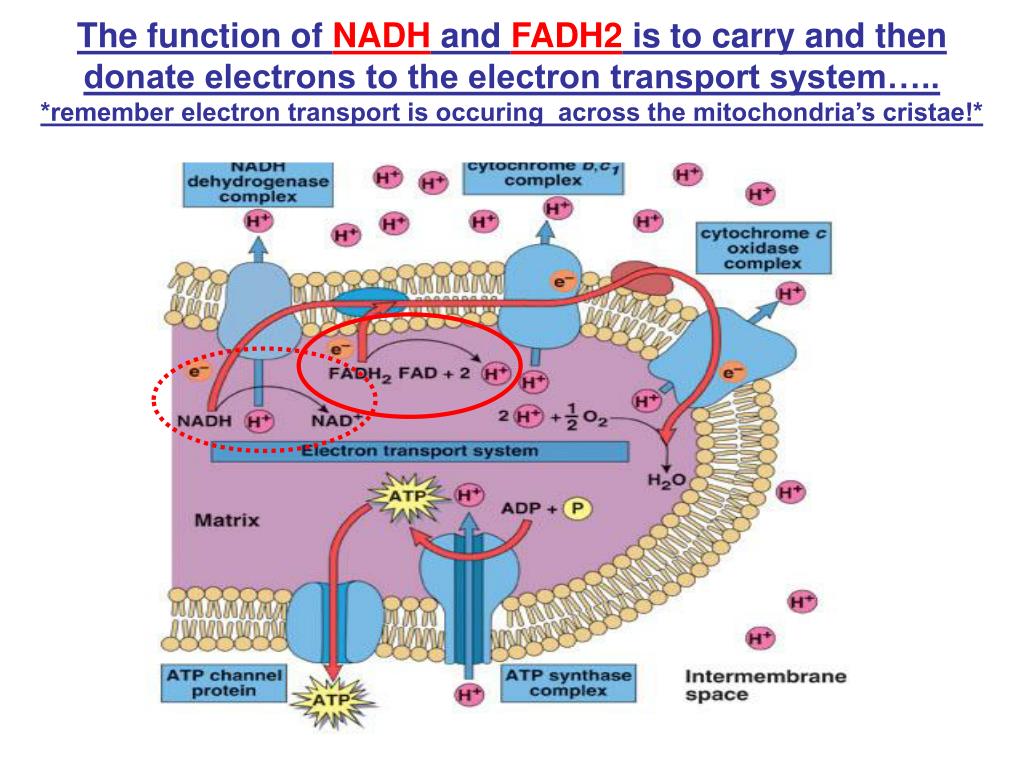
How Does Nadh And Fadh2 Donate Electrons To The Electron Transport Chain
Iron Electron Donor This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. Lithotrophs have been found growing in rock formations. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electron Donor Lithotrophs have been found growing in rock formations. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form of electrotrophy is fe. Iron Electron Donor.
From www.researchgate.net
Schematic representation of the relationship between a metal catalyst Iron Electron Donor Electron flow in these organisms is. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and. Iron Electron Donor.
From pubs.acs.org
Dual Role of Humic Substances As Electron Donor and Shuttle for Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. This is explained. Iron Electron Donor.
From donate-faqs.com
How Does Nadh And Fadh2 Donate Electrons To The Electron Transport Chain Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. Electron flow in these organisms is. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient. Iron Electron Donor.
From www.slideserve.com
PPT Electron Transport Chain/Respiratory Chain PowerPoint Iron Electron Donor Electron flow in these organisms is. Lithotrophs have been found growing in rock formations. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. This is explained. Iron Electron Donor.
From material-properties.org
Iron Periodic Table and Atomic Properties Iron Electron Donor This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron. Iron Electron Donor.
From www.researchgate.net
Screening of different redoxins as electron donors for RNR. All six Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing. Iron Electron Donor.
From www.nuclear-power.com
Iron Electron Affinity Electronegativity Ionization Energy of Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. This is explained by the needs of both the ferrous iron. Iron Electron Donor.
From www.alamy.com
Fe Iron, Periodic Table of the Elements, Shell Structure of Iron Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the. Iron Electron Donor.
From www.researchgate.net
The diversity of electron donors and electron acceptors used by life Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations. Electron flow in these organisms is. This is explained. Iron Electron Donor.
From www.sciencephoto.com
Iron, atomic structure Stock Image C013/1539 Science Photo Library Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure. Iron Electron Donor.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to.. Iron Electron Donor.
From www.sciencephoto.com
Iron, atomic structure Stock Image C018/3707 Science Photo Library Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing in rock formations. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and. Iron Electron Donor.
From www.researchgate.net
Scheme for Fe(III)/d 5 photoredox activation of electron donors (D) and Iron Electron Donor Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Lithotrophs have been found growing in rock formations. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained. Iron Electron Donor.
From valenceelectrons.com
How to Find the Valence Electrons for Iron (Fe)? Iron Electron Donor Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form. Iron Electron Donor.
From www.mdpi.com
Free FullText Iron Compounds in Anaerobic Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing in rock formations. The most intensively studied form of electrotrophy is fe. Iron Electron Donor.
From www.researchgate.net
Different strategies for the design of CO 2 reduction catalysts. Chang Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form. Iron Electron Donor.
From sml.snl.no
mitokondrie Store medisinske leksikon Iron Electron Donor This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have. Iron Electron Donor.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Lithotrophs have been found growing in rock formations. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form. Iron Electron Donor.
From anelementaday.wordpress.com
Day 3 Iron An Element A Day Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations. The most intensively studied form of electrotrophy is fe (ii) oxidation. Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. This is explained. Iron Electron Donor.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms.. Iron Electron Donor.
From rapidelectron.blogspot.com
Electron Configuration For An Atom Of Iron Rapid Electron Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron. Iron Electron Donor.
From www.cell.com
Bioenergetic challenges of microbial iron metabolisms Trends in Iron Electron Donor Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and. Iron Electron Donor.
From www.youtube.com
A stepbystep description of how to write the electron configuration Iron Electron Donor Electron flow in these organisms is. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Lithotrophs have been found growing in rock formations. The most intensively studied form of electrotrophy is fe (ii) oxidation. This is explained. Iron Electron Donor.
From www.researchgate.net
Examples of mechanisms of electron transfer. H2 transfer between P Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure. Iron Electron Donor.
From www.bigstockphoto.com
3d Render Atom Structure Iron Image & Photo Bigstock Iron Electron Donor Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a. Iron Electron Donor.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Electron Donor This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Electron flow in these organisms is. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. The most intensively studied form of electrotrophy is fe (ii) oxidation. Ferric iron (fe 3+) is a. Iron Electron Donor.
From www.istockphoto.com
Fe Iron Element Information Facts Properties Trends Uses And Comparison Iron Electron Donor Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. The most intensively studied form of electrotrophy is fe (ii) oxidation. Electron flow in these organisms is. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Lithotrophs have been found growing in. Iron Electron Donor.
From www.youtube.com
How to find Protons & Electrons for Fe2+ and Fe3+ (Iron II and III ions Iron Electron Donor Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. The most intensively studied form of electrotrophy is fe (ii) oxidation.. Iron Electron Donor.
From new--electronic.blogspot.com
Electron Donors List Iron Electron Donor Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Electron flow in these organisms is. Lithotrophs have been found growing in rock formations. The most intensively studied form of electrotrophy is fe (ii) oxidation. This is explained. Iron Electron Donor.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Iron (Fe) YouTube Iron Electron Donor The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. Ferric iron (fe 3+). Iron Electron Donor.
From www.shutterstock.com
18,716 Electrons metal atoms Images, Stock Photos & Vectors Shutterstock Iron Electron Donor Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained. Iron Electron Donor.
From valenceelectrons.com
How Many Valence Electrons Does Iron (Fe) Have? Iron Electron Donor Lithotrophs have been found growing in rock formations. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Electron flow in these organisms is. The most intensively studied form of electrotrophy is fe (ii) oxidation. Inorganic electron donors include hydrogen, carbon monoxide, ammonia, nitrite, sulfur, sulfide, and ferrous iron. This is explained. Iron Electron Donor.
From www.researchgate.net
Model of Fe(II) oxidation electron transport pathway of A. ferrooxidans Iron Electron Donor Lithotrophs have been found growing in rock formations. This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms. Electron flow in these organisms is. The most intensively studied form. Iron Electron Donor.
From www.science.org
The ElectronPairing Mechanism of IronBased Superconductors Science Iron Electron Donor This is explained by the needs of both the ferrous iron [fe(ii)] oxidizer and ferric iron [fe(iii)] reducer to have sufficient exposure to. The most intensively studied form of electrotrophy is fe (ii) oxidation. Lithotrophs have been found growing in rock formations. Ferric iron (fe 3+) is a widespread anaerobic terminal electron acceptor used by both autotrophic and heterotrophic organisms.. Iron Electron Donor.