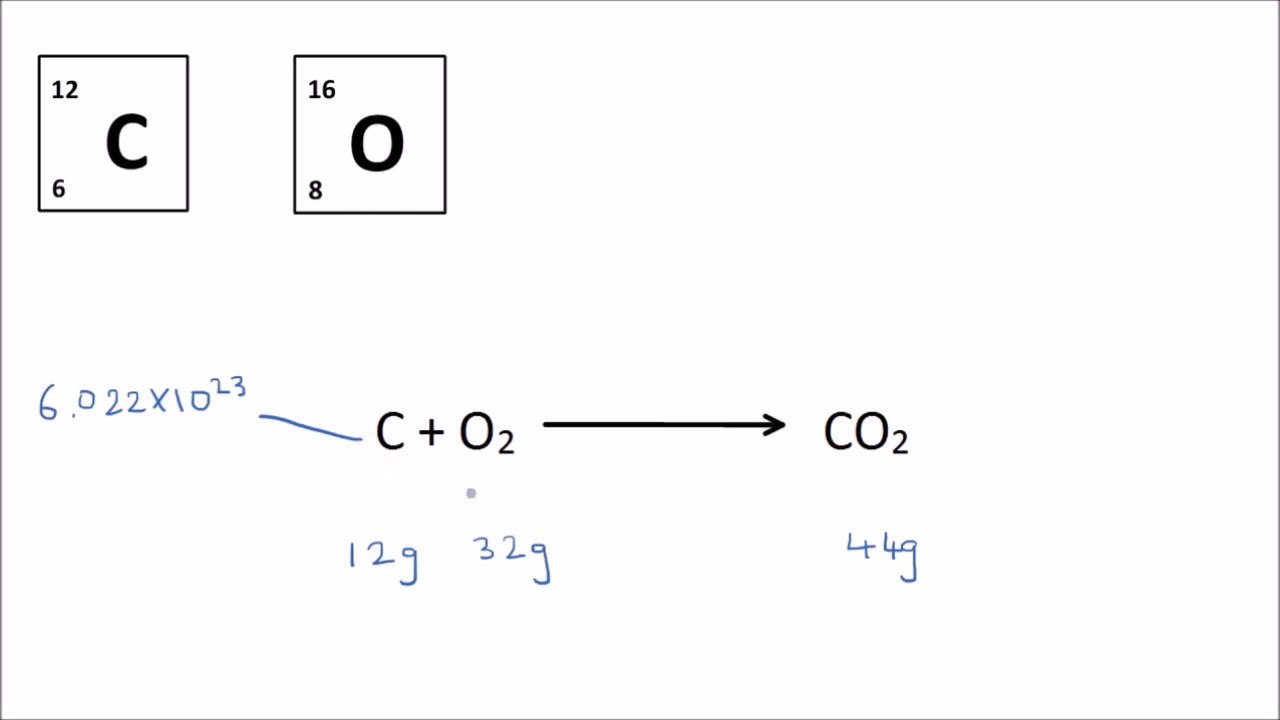
The Mole Explained . This quantity is sometimes referred. The number of particles in a substance can be found using the avogadro constant. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole is the unit of measurement in the international system of units (si) for amount of substance. The mole unit describes the amount or number of things. One mole is exactly 6.02214076×10 23 particles. The mole is an si unit used to measure the amount of any substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole is the unit for the amount of substance. In chemistry, a mole is an si base unit for quantity. The abbreviation for mole is mol.
from www.youtube.com
One mole is exactly 6.02214076×10 23 particles. In chemistry, a mole is an si base unit for quantity. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole is the unit of measurement in the international system of units (si) for amount of substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The abbreviation for mole is mol. The mole is an si unit used to measure the amount of any substance. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole unit describes the amount or number of things. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance.
Moles Explained GCSE Science Chemistry Get To Know Science YouTube
The Mole Explained The mole is the unit of measurement in the international system of units (si) for amount of substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole unit describes the amount or number of things. In chemistry, a mole is an si base unit for quantity. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole is an si unit used to measure the amount of any substance. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The abbreviation for mole is mol. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole is the unit of measurement in the international system of units (si) for amount of substance. The mole is the unit for the amount of substance. One mole is exactly 6.02214076×10 23 particles. The number of particles in a substance can be found using the avogadro constant. This quantity is sometimes referred.
From www.slideserve.com
PPT The Mole Concept PowerPoint Presentation, free download ID4787514 The Mole Explained Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. One mole is exactly 6.02214076×10 23 particles. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The number of particles in a substance can be found using the. The Mole Explained.
From studylib.net
The Mole Explained! The Mole Explained In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. One mole is exactly 6.02214076×10 23 particles. In chemistry, a mole is an si base unit for quantity. The number of particles in a substance can be found using the avogadro constant. The mole. The Mole Explained.
From www.slideserve.com
PPT The Mole Concept PowerPoint Presentation, free download ID1972513 The Mole Explained One mole is exactly 6.02214076×10 23 particles. In chemistry, a mole is an si base unit for quantity. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The mole unit describes the amount or number of things. This quantity is sometimes referred. Despite. The Mole Explained.
From www.youtube.com
Mole Concept Explained (Quick Chemistry Review) YouTube The Mole Explained The mole is an si unit used to measure the amount of any substance. The abbreviation for mole is mol. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see. The Mole Explained.
From www.youtube.com
THE MOLE CONCEPT EXPLAINED I WHAT IS A MOLE? VISUALIZED VIA A CHART The Mole Explained Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The abbreviation for mole is mol. One mole is exactly 6.02214076×10 23 particles. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as. The Mole Explained.
From www.youtube.com
What is mole? 11th Chemistry Explanation in தமிழ் YouTube The Mole Explained This quantity is sometimes referred. In chemistry, a mole is an si base unit for quantity. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole is the unit for the amount of substance. The number of particles in a substance can be found using the. The Mole Explained.
From learningwohrleef.z14.web.core.windows.net
Avogadro's Number And The Mole The Mole Explained The abbreviation for mole is mol. The mole is the unit for the amount of substance. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of. The Mole Explained.
From ar.inspiredpencil.com
Mole Chemistry The Mole Explained This quantity is sometimes referred. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole unit describes the amount or number of things. The mole is the unit of measurement in the international system of units (si) for amount of substance. In chemistry, a mole is. The Mole Explained.
From www.docbrown.info
definition mole explained molar mass mol mols calculations how to read The Mole Explained Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole unit describes the amount or number of things. The number of particles in a substance can be found using the avogadro constant. The mole is an si unit used to measure the amount of any substance. The abbreviation for. The Mole Explained.
From www.youtube.com
The mole explained Chemistry at heart YouTube The Mole Explained In chemistry, a mole is an si base unit for quantity. One mole is exactly 6.02214076×10 23 particles. The mole unit describes the amount or number of things. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. In chemistry the mole is a fundamental unit in the système international d'unités,. The Mole Explained.
From wwwaboutmole.blogspot.com
Mole and Molarity Mole Concept The Mole Explained The number of particles in a substance can be found using the avogadro constant. The mole is the unit of measurement in the international system of units (si) for amount of substance. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well,. The Mole Explained.
From www.youtube.com
How to Use the MOLE in Chemistry YouTube The Mole Explained Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole unit describes the amount or number of things. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on. The Mole Explained.
From www.youtube.com
Moles Moles to Grams Conversions The Mole Simply Explained With The Mole Explained The mole is the unit for the amount of substance. In chemistry, a mole is an si base unit for quantity. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The abbreviation for mole is mol. This quantity is sometimes referred. Mole, in chemistry, a standard scientific. The Mole Explained.
From www.slideserve.com
PPT Ch. 6 Notes Chemical Composition What is a mole? PowerPoint The Mole Explained In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The mole is the unit for the amount of substance. The mole is an si unit used to measure the amount of any substance. Despite the name, it has nothing to do with the. The Mole Explained.
From www.youtube.com
The Mole Explained YouTube The Mole Explained This quantity is sometimes referred. The abbreviation for mole is mol. The mole is an si unit used to measure the amount of any substance. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The number of particles in a substance can be found using the avogadro. The Mole Explained.
From chemistnotes.com
Introduction to mole concept Explained with examples Chemistry Notes The Mole Explained This quantity is sometimes referred. One mole is exactly 6.02214076×10 23 particles. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole unit describes the amount or number of things. The number of particles in a substance can be found using the avogadro constant. The abbreviation. The Mole Explained.
From www.youtube.com
What is a Chemistry Mole....Explained! YouTube The Mole Explained One mole is exactly 6.02214076×10 23 particles. The number of particles in a substance can be found using the avogadro constant. The mole is an si unit used to measure the amount of any substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. Presented by msnbc host alex wagner, this. The Mole Explained.
From www.youtube.com
Chemistry Mole Concept YouTube The Mole Explained One mole is exactly 6.02214076×10 23 particles. The number of particles in a substance can be found using the avogadro constant. The mole unit describes the amount or number of things. The abbreviation for mole is mol. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the. The Mole Explained.
From www.youtube.com
Avogadro’s Number & The Mole Explained! YouTube The Mole Explained The abbreviation for mole is mol. The number of particles in a substance can be found using the avogadro constant. The mole is an si unit used to measure the amount of any substance. In chemistry, a mole is an si base unit for quantity. The mole is the unit of measurement in the international system of units (si) for. The Mole Explained.
From www.youtube.com
Concept of Mole explained too good,,,,,,,,, YouTube The Mole Explained In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The mole is the unit of measurement in the international system of units (si) for amount of substance. The number of particles in a substance can be found using the avogadro constant. The mole. The Mole Explained.
From www.slideserve.com
PPT The Mole Road Map PowerPoint Presentation, free download ID6150660 The Mole Explained The number of particles in a substance can be found using the avogadro constant. In chemistry, a mole is an si base unit for quantity. The mole unit describes the amount or number of things. This quantity is sometimes referred. The abbreviation for mole is mol. In chemistry the mole is a fundamental unit in the système international d'unités, the. The Mole Explained.
From www.slideserve.com
PPT Chapter 6 Moles, Molar Mass, Percent Composition and Formulas The Mole Explained Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The mole is the unit for the amount of substance. In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The mole. The Mole Explained.
From www.domyown.com
What Does A Mole Look Like Ground Mole Identification Guide The Mole Explained Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole unit describes the amount or number. The Mole Explained.
From www.slideserve.com
PPT Chemistry 20 Mole Conversions PowerPoint Presentation, free The Mole Explained Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole is the unit of measurement in the international system of units (si) for amount of substance. In chemistry, a mole is an si base unit for quantity. Despite the name, it has nothing to do with the small brown/gray. The Mole Explained.
From www.youtube.com
Understanding Moles (GCSE Chemistry) YouTube The Mole Explained The mole is an si unit used to measure the amount of any substance. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole is the unit of measurement in the international system of units (si) for amount. The Mole Explained.
From www.compoundchem.com
Mole Day Avogadro & The Mole Compound Interest The Mole Explained Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. The mole unit describes the amount or number of things. The abbreviation for mole is mol. The number of particles in a substance can be found using the avogadro constant. Despite the name, it has nothing to do with the small. The Mole Explained.
From chemistnotes.com
Introduction to mole concept Explained with examples Chemistry Notes The Mole Explained The mole unit describes the amount or number of things. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. In. The Mole Explained.
From www.youtube.com
Moles Explained GCSE Science Chemistry Get To Know Science YouTube The Mole Explained In chemistry the mole is a fundamental unit in the système international d'unités, the si system, and it is used to measure the amount of substance. The mole is the unit for the amount of substance. One mole is exactly 6.02214076×10 23 particles. The mole is an si unit used to measure the amount of any substance. Mole, in chemistry,. The Mole Explained.
From sciencenotes.org
Moles to Grams Conversion Examples The Mole Explained The mole unit describes the amount or number of things. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well,. The Mole Explained.
From www.youtube.com
What is a mole? A Level Physics YouTube The Mole Explained The number of particles in a substance can be found using the avogadro constant. The mole is the unit for the amount of substance. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole is the unit of. The Mole Explained.
From mungfali.com
The Mole Chemistry The Mole Explained The mole unit describes the amount or number of things. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well,. The Mole Explained.
From periodictable.me
Introduction to the Mole in Chemistry A Brief Explanation The Mole Explained This quantity is sometimes referred. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. The abbreviation for mole is mol. The number of particles in a substance can be found using the avogadro constant. In chemistry, a mole is an si base unit for quantity. The mole. The Mole Explained.
From surfguppy.com
The Mole Concept Surfguppy Chemistry made easy visual learning The Mole Explained In chemistry, a mole is an si base unit for quantity. Mole, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules,. One mole is exactly 6.02214076×10 23 particles. The mole is the unit for the amount of substance. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit. The Mole Explained.
From www.youtube.com
Concept of Mole Part 1 Atoms and Molecules Infinity Learn YouTube The Mole Explained The mole is an si unit used to measure the amount of any substance. The number of particles in a substance can be found using the avogadro constant. The mole is the unit for the amount of substance. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food.. The Mole Explained.
From www.lessonplanet.com
The Mole Concept PPT for 8th 12th Grade Lesson The Mole Explained The abbreviation for mole is mol. Despite the name, it has nothing to do with the small brown/gray garden pest or the tasty chocolate sauce on mexican food. Presented by msnbc host alex wagner, this revival of the 2000s broadcast hit (which featured a young anderson cooper) will see a group of 12 players — well, actually, 11. The mole. The Mole Explained.