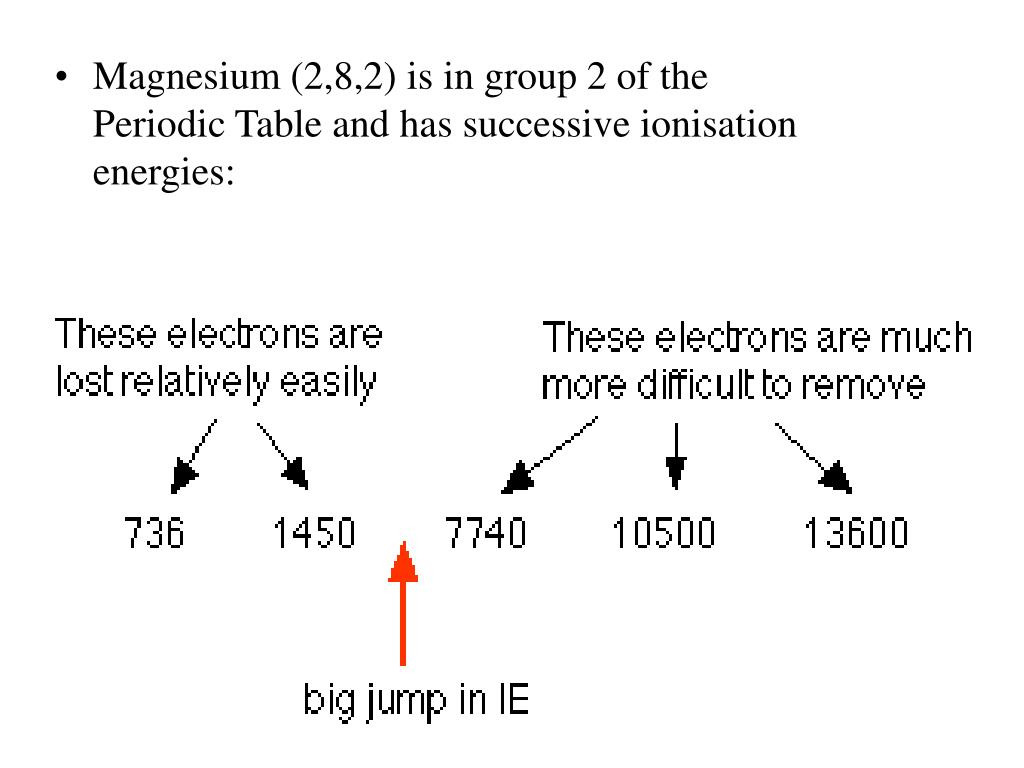
Magnesium Higher Ionisation Energy Than Strontium . First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. These tables list values of molar ionization energies, measured in kj⋅mol −1. Notice how the second ionization energy for sodium, third ionization energy. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Compare magnesium and strontium on the. Molar ionization energies of the elements. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. This is the energy per.
from www.slideserve.com
Molar ionization energies of the elements. This is the energy per. 120 rows ionization energy chart of all the elements is given below. Notice how the second ionization energy for sodium, third ionization energy. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. Compare magnesium and strontium on the. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium.
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142
Magnesium Higher Ionisation Energy Than Strontium These tables list values of molar ionization energies, measured in kj⋅mol −1. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Compare magnesium and strontium on the. This is the energy per. First ionization energy, second ionization energy as well as. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1.
From slideplayer.com
Higher Chemistry Periodicity ppt download Magnesium Higher Ionisation Energy Than Strontium Notice how the second ionization energy for sodium, third ionization energy. These tables list values of molar ionization energies, measured in kj⋅mol −1. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Compare magnesium and strontium on. Magnesium Higher Ionisation Energy Than Strontium.
From ar.inspiredpencil.com
Periodic Table Ionization Energy Labeled Magnesium Higher Ionisation Energy Than Strontium This is the energy per. 120 rows ionization energy chart of all the elements is given below. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Compare magnesium and strontium on the. First ionization energy, second ionization energy as well as. Molar ionization energies of the elements.. Magnesium Higher Ionisation Energy Than Strontium.
From www.chemistrylearner.com
Ionization Energy Definition, Chart & Periodic Table Trend Magnesium Higher Ionisation Energy Than Strontium Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy, second ionization energy as well as. Molar ionization energies of the elements. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy. Magnesium Higher Ionisation Energy Than Strontium.
From www.numerade.com
SOLVEDMagnesium has a higher first ionization energy than sodium Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. Molar ionization energies of the elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the. Magnesium Higher Ionisation Energy Than Strontium.
From slideplayer.com
Higher Chemistry Periodicity ppt download Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. This is the energy per. First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove. Magnesium Higher Ionisation Energy Than Strontium.
From ar.inspiredpencil.com
Periodic Table Ionization Energy 3d Magnesium Higher Ionisation Energy Than Strontium Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. This is the energy per. First ionization energy, second ionization energy as well as. Notice how the second ionization energy for sodium, third ionization energy. Molar ionization energies of the elements. 120 rows ionization energy chart of all. Magnesium Higher Ionisation Energy Than Strontium.
From www.numerade.com
SOLVED 'Rank these elements according to first ionization energy Magnesium Higher Ionisation Energy Than Strontium These tables list values of molar ionization energies, measured in kj⋅mol −1. Compare magnesium and strontium on the. This is the energy per. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its. Magnesium Higher Ionisation Energy Than Strontium.
From www.geeksforgeeks.org
Ionization Energy Definition, Formulas, and Solved Examples Magnesium Higher Ionisation Energy Than Strontium This is the energy per. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Compare magnesium and strontium on the. 120 rows ionization energy chart of all the elements is given below.. Magnesium Higher Ionisation Energy Than Strontium.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the. Magnesium Higher Ionisation Energy Than Strontium.
From www.youtube.com
First and second ionisation energies of magnesium are `7.646 eV` and Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. 120 rows ionization energy chart of all the elements is given below. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as. Magnesium Higher Ionisation Energy Than Strontium.
From chemistnotes.com
Periodic table Archives Chemistry Notes Magnesium Higher Ionisation Energy Than Strontium This is the energy per. Molar ionization energies of the elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and. Magnesium Higher Ionisation Energy Than Strontium.
From www.slideserve.com
PPT Chemistry Chapter 5 PowerPoint Presentation, free download ID Magnesium Higher Ionisation Energy Than Strontium These tables list values of molar ionization energies, measured in kj⋅mol −1. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Molar ionization energies of the elements. 120 rows ionization energy chart of all the elements is given below. Table \(\pageindex{1}\) gives the ionization energies for the. Magnesium Higher Ionisation Energy Than Strontium.
From www.numerade.com
SOLVED Rank the following elements according to their ionization Magnesium Higher Ionisation Energy Than Strontium Notice how the second ionization energy for sodium, third ionization energy. First ionization energy, second ionization energy as well as. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Compare magnesium and strontium on the. Molar ionization energies of the elements. These tables list values of molar. Magnesium Higher Ionisation Energy Than Strontium.
From www.sliderbase.com
Periodic Trends Presentation Chemistry Magnesium Higher Ionisation Energy Than Strontium This is the energy per. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1. Compare magnesium and strontium. Magnesium Higher Ionisation Energy Than Strontium.
From www.slideserve.com
PPT Chemistry UNIT 3 PowerPoint Presentation, free download ID5906142 Magnesium Higher Ionisation Energy Than Strontium First ionization energy, second ionization energy as well as. Molar ionization energies of the elements. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. 120 rows ionization energy chart of. Magnesium Higher Ionisation Energy Than Strontium.
From www.reddit.com
why is magnesium have higher energy than sodium? It's first ionisation Magnesium Higher Ionisation Energy Than Strontium This is the energy per. Compare magnesium and strontium on the. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. 120 rows ionization energy chart of all the elements is given below. Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed. Magnesium Higher Ionisation Energy Than Strontium.
From slideplayer.com
Trends in Periodic Table ppt download Magnesium Higher Ionisation Energy Than Strontium 120 rows ionization energy chart of all the elements is given below. Molar ionization energies of the elements. Notice how the second ionization energy for sodium, third ionization energy. This is the energy per. Compare magnesium and strontium on the. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from. Magnesium Higher Ionisation Energy Than Strontium.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID753034 Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. Molar ionization energies of the. Magnesium Higher Ionisation Energy Than Strontium.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Magnesium Higher Ionisation Energy Than Strontium Molar ionization energies of the elements. Notice how the second ionization energy for sodium, third ionization energy. This is the energy per. These tables list values of molar ionization energies, measured in kj⋅mol −1. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed. Magnesium Higher Ionisation Energy Than Strontium.
From www.numerade.com
SOLVED Using only the periodic table arrange the following elements in Magnesium Higher Ionisation Energy Than Strontium Table \(\pageindex{1}\) gives the ionization energies for the period three elements. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Compare magnesium and strontium on the. 120 rows ionization energy chart of. Magnesium Higher Ionisation Energy Than Strontium.
From www.researchgate.net
Energylevel diagram for magnesium showing the levels relevant for this Magnesium Higher Ionisation Energy Than Strontium Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. First ionization energy, second ionization energy as well as. This is the energy per. Molar ionization energies of the elements. These. Magnesium Higher Ionisation Energy Than Strontium.
From byjus.com
Magnesium has the firšt and second ionizatio potential 7.646 and 15. Magnesium Higher Ionisation Energy Than Strontium Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Molar ionization energies of the elements. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. 120 rows ionization energy chart of all the elements is given below. Aluminum and gallium,. Magnesium Higher Ionisation Energy Than Strontium.
From chemwiki.ucdavis.edu
Periodic Properties of the Elements Chemwiki Magnesium Higher Ionisation Energy Than Strontium Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Compare magnesium and strontium on the. First ionization energy, second ionization energy as well as. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Chemists define the ionization energy (\(i\)) of an element as the amount. Magnesium Higher Ionisation Energy Than Strontium.
From www.researchgate.net
Energylevel diagram for magnesium. The full length horizontal lines Magnesium Higher Ionisation Energy Than Strontium Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. These tables list values of molar ionization energies, measured in kj⋅mol −1. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. 120 rows ionization energy chart of all the elements is given below. Compare magnesium and. Magnesium Higher Ionisation Energy Than Strontium.
From slideplayer.com
Trends in Periodic Table ppt download Magnesium Higher Ionisation Energy Than Strontium This is the energy per. Notice how the second ionization energy for sodium, third ionization energy. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Molar ionization energies of the elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to. Magnesium Higher Ionisation Energy Than Strontium.
From www.slideserve.com
PPT Chapter 7 PowerPoint Presentation, free download ID5457348 Magnesium Higher Ionisation Energy Than Strontium Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. 120 rows ionization energy chart of all the elements is. Magnesium Higher Ionisation Energy Than Strontium.
From www.chemistrylearner.com
Periodic Trends Definition and Properties Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. First ionization energy, second ionization energy as well as. This is the energy per. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. 120 rows ionization energy chart of all the elements is given below.. Magnesium Higher Ionisation Energy Than Strontium.
From www.periodic-table.org
Magnesium Ionization Energy Magnesium Higher Ionisation Energy Than Strontium Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. This is the energy per. First ionization energy, second ionization energy as well as. Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared. Magnesium Higher Ionisation Energy Than Strontium.
From abbiebolton.z21.web.core.windows.net
Successive Ionization Energies Chart Magnesium Higher Ionisation Energy Than Strontium Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Compare magnesium and strontium on the. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. Notice how the second ionization energy for sodium, third ionization energy. Molar ionization energies of. Magnesium Higher Ionisation Energy Than Strontium.
From www.toppr.com
First and second ionisation energies of magnesium are 7.646 and 15 Magnesium Higher Ionisation Energy Than Strontium Compare magnesium and strontium on the. These tables list values of molar ionization energies, measured in kj⋅mol −1. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy, second ionization energy as well as. This is the energy per.. Magnesium Higher Ionisation Energy Than Strontium.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Magnesium Higher Ionisation Energy Than Strontium Notice how the second ionization energy for sodium, third ionization energy. Table \(\pageindex{1}\) gives the ionization energies for the period three elements. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy, second ionization energy as well as. 120. Magnesium Higher Ionisation Energy Than Strontium.
From www.numerade.com
Using only the periodic table, arrange the following elements in order Magnesium Higher Ionisation Energy Than Strontium Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. This is the energy per. 120 rows ionization energy chart of all the elements is given below. Aluminum and gallium, both in the same group as boron, similarly show a decrease in. Magnesium Higher Ionisation Energy Than Strontium.
From www.slideserve.com
PPT Ionization Energy PowerPoint Presentation, free download ID2629738 Magnesium Higher Ionisation Energy Than Strontium Molar ionization energies of the elements. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. This is the energy per. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state.. Magnesium Higher Ionisation Energy Than Strontium.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Magnesium Higher Ionisation Energy Than Strontium Aluminum and gallium, both in the same group as boron, similarly show a decrease in ionization energy compared to magnesium and calcium. First ionization energy, second ionization energy as well as. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. This. Magnesium Higher Ionisation Energy Than Strontium.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Higher Ionisation Energy Than Strontium Notice how the second ionization energy for sodium, third ionization energy. Chemists define the ionization energy (\(i\)) of an element as the amount of energy needed to remove an electron from the gaseous atom \(e\) in its ground state. First ionization energy, second ionization energy as well as. Compare magnesium and strontium on the. These tables list values of molar. Magnesium Higher Ionisation Energy Than Strontium.