Copper Chloride Equilibrium . While cu 2+ (aq) ions are blue, the resulting product is green. This is known as le chatelier’s. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Figure \(\pageindex{2}\) compares three aqueous copper complexes. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,.
from www.numerade.com
Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. Figure \(\pageindex{2}\) compares three aqueous copper complexes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. This is known as le chatelier’s. While cu 2+ (aq) ions are blue, the resulting product is green. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced.
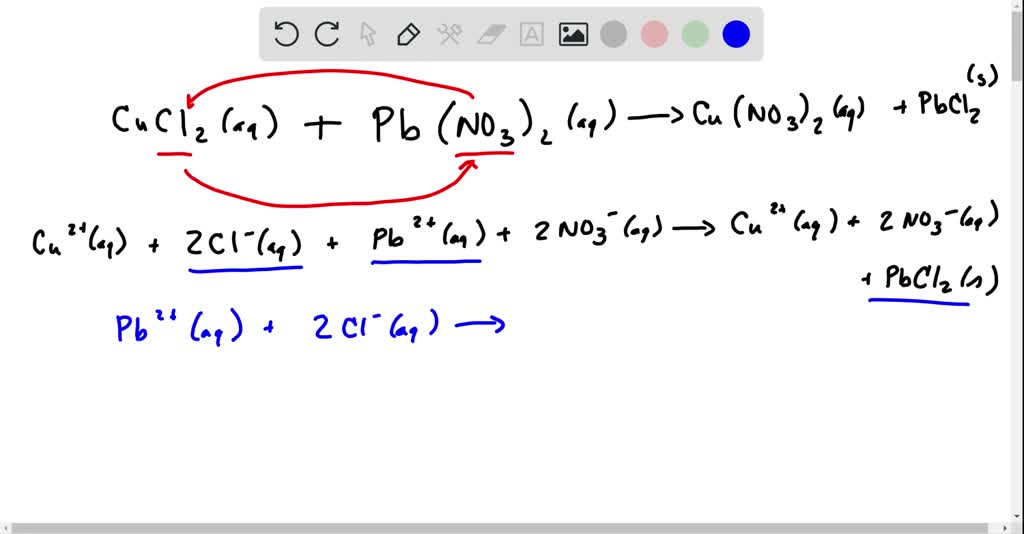
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous
Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. Figure \(\pageindex{2}\) compares three aqueous copper complexes. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. While cu 2+ (aq) ions are blue, the resulting product is green. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. This is known as le chatelier’s. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced.
From www.youtube.com
Electrolysis of Copper Chloride solution National 5 YouTube Copper Chloride Equilibrium In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. While cu 2+ (aq) ions are blue, the resulting product is green. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced.. Copper Chloride Equilibrium.
From www.numerade.com
SOLVED What are the equilibrium concentrations of Cu+ and Cl in a Copper Chloride Equilibrium To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Figure \(\pageindex{2}\) compares three aqueous copper complexes. While cu. Copper Chloride Equilibrium.
From www.youtube.com
Metal complexes 12. Reversible ligand substitution with CuCl2 YouTube Copper Chloride Equilibrium In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. This is known as le chatelier’s. While cu 2+ (aq) ions are. Copper Chloride Equilibrium.
From www.numerade.com
⏩SOLVEDCopper(Il) chloride and lead(II) nitrate react in aqueous Copper Chloride Equilibrium When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. Investigate the shifts. Copper Chloride Equilibrium.
From www.semanticscholar.org
Figure 1 from The Equilibrium Molecular Structure of Cyclic (Alkyl Copper Chloride Equilibrium In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. This is known as le chatelier’s. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. To make the equilibrium mixture i. Copper Chloride Equilibrium.
From www.slideserve.com
PPT Chapter 11 PowerPoint Presentation, free download ID235007 Copper Chloride Equilibrium Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Figure \(\pageindex{2}\) compares three aqueous copper complexes. Investigate the shifts that can be observed by applying a stress to small amounts. Copper Chloride Equilibrium.
From askfilo.com
Calculate equilibrium constant for the disproportionation reaction Copper Chloride Equilibrium Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. In this section we will apply chemical equilibria to the. Copper Chloride Equilibrium.
From slideplayer.com
Equilibrium Systems Chapter ppt download Copper Chloride Equilibrium This is known as le chatelier’s. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to. Copper Chloride Equilibrium.
From www.slideserve.com
PPT Naming Multivalent Compounds PowerPoint Presentation, free Copper Chloride Equilibrium Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. To make the equilibrium mixture i give to students, i use some copper ii. Copper Chloride Equilibrium.
From www.researchgate.net
6. Solubility versus pH curves for the thermodynamically stable Copper Chloride Equilibrium To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Le chatelier's principle. Copper Chloride Equilibrium.
From www.researchgate.net
3 the steady equilibrium temperature of red copper bare wire with Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. This is known as le chatelier’s. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. Sample x was determined to be 0.15 m. Copper Chloride Equilibrium.
From www.numerade.com
SOLVED What are the equilibrium concentrations of Cu+ and Cl in a Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced.. Copper Chloride Equilibrium.
From www.semanticscholar.org
Figure 2 from The Equilibrium Molecular Structure of Cyclic (Alkyl Copper Chloride Equilibrium Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. Figure \(\pageindex{2}\) compares three aqueous copper complexes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. When cucl 2 is dissolved in h 2 o, a beautiful green color due. Copper Chloride Equilibrium.
From www.thesciencehive.co.uk
Reversible Reactions and Equilibria (GCSE) — the science sauce Copper Chloride Equilibrium Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. To make the equilibrium mixture i give to students, i use some copper ii chloride,. Copper Chloride Equilibrium.
From www.researchgate.net
Partial clarification of copper chloride. Top spectra is copper Copper Chloride Equilibrium While cu 2+ (aq) ions are blue, the resulting product is green. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. To make the. Copper Chloride Equilibrium.
From www.researchgate.net
Electronic absorption spectra of a 0.01 M solution of copper (II Copper Chloride Equilibrium Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Figure \(\pageindex{2}\) compares three aqueous copper complexes. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium. Copper Chloride Equilibrium.
From www.numerade.com
SOLVED What are the equilibrium concentrations of Cu+ and Cl in a Copper Chloride Equilibrium To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. When cucl 2 is dissolved in h 2 o,. Copper Chloride Equilibrium.
From www.youtube.com
Electrolysis of copper(II) chloride YouTube Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. While cu 2+ (aq) ions are blue, the resulting product is green. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly. Copper Chloride Equilibrium.
From www.researchgate.net
The fugacity of copper chlorides in H 2 O/HCl gas mixtures in Copper Chloride Equilibrium Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. In this section we will apply chemical equilibria to the concept of solubility and introduce a. Copper Chloride Equilibrium.
From www.numerade.com
What are the equilibrium concentrations of Cu+ and Cl in a saturated Copper Chloride Equilibrium In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. While cu 2+ (aq) ions are blue, the resulting product is green. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium. Copper Chloride Equilibrium.
From www.youtube.com
copper chloride equilibrium YouTube Copper Chloride Equilibrium When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. Le chatelier's principle states that when a system in chemical equilibrium is disturbed. Copper Chloride Equilibrium.
From www.researchgate.net
Pourbaix diagram for the copperwaterchloride system at 25°C for a Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl.. Copper Chloride Equilibrium.
From www.researchgate.net
(PDF) The Equilibrium Molecular Structure of Cyclic (Alkyl)(Amino Copper Chloride Equilibrium While cu 2+ (aq) ions are blue, the resulting product is green. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. This is known as le chatelier’s. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2. Copper Chloride Equilibrium.
From www.doubtnut.com
How the following conversions are made? (a). Copper chloride from co Copper Chloride Equilibrium When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Investigate the shifts that can be observed by applying a stress to. Copper Chloride Equilibrium.
From www.numerade.com
SOLVEDA Copper(I) ion disproportionates to copper metal and copper(II Copper Chloride Equilibrium In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Investigate the shifts that can be observed by applying a stress to. Copper Chloride Equilibrium.
From www.numerade.com
SOLVED The solubility equilibrium constant for the dissolution of Copper Chloride Equilibrium When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. Investigate the shifts that can be observed by applying a stress to. Copper Chloride Equilibrium.
From www.researchgate.net
Distribution of copper species as a function of chloride concentration Copper Chloride Equilibrium This is known as le chatelier’s. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a. Copper Chloride Equilibrium.
From mmerevise.co.uk
Reactions of Acids Worksheets and Revision MME Copper Chloride Equilibrium Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Investigate the shifts that can be observed by applying a stress to small amounts of. Copper Chloride Equilibrium.
From www.semanticscholar.org
Table 1 from The Equilibrium Molecular Structure of Cyclic (Alkyl Copper Chloride Equilibrium Figure \(\pageindex{2}\) compares three aqueous copper complexes. In this section we will apply chemical equilibria to the concept of solubility and introduce a type of equilibrium constant, the. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. This is. Copper Chloride Equilibrium.
From www.researchgate.net
5 Solubility of copper(II) in chloride solutions at 50 °C. • CuCl Copper Chloride Equilibrium Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Figure \(\pageindex{2}\) compares three aqueous copper complexes. Le chatelier's principle states that when a system in chemical equilibrium is disturbed by a change in temperature,. To make the equilibrium mixture i give to students,. Copper Chloride Equilibrium.
From www.animalia-life.club
Copper Chloride Copper Chloride Equilibrium While cu 2+ (aq) ions are blue, the resulting product is green. Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Figure \(\pageindex{2}\). Copper Chloride Equilibrium.
From www.researchgate.net
Water vapor absorption curves of the three (A) copper chloride, (B Copper Chloride Equilibrium When cucl 2 is dissolved in h 2 o, a beautiful green color due mainly to the complex [cucl 2 (h 2 o) 2 ] is produced. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. Le chatelier's principle states that when a. Copper Chloride Equilibrium.
From www.semanticscholar.org
Figure 5 from The Equilibrium Molecular Structure of Cyclic (Alkyl Copper Chloride Equilibrium Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a bit more. When cucl 2 is. Copper Chloride Equilibrium.
From www.youtube.com
Cobalt Chloride Equilibrium // HSC Chemistry YouTube Copper Chloride Equilibrium Investigate the shifts that can be observed by applying a stress to small amounts of this solution in test tubes. While cu 2+ (aq) ions are blue, the resulting product is green. This is known as le chatelier’s. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2. Copper Chloride Equilibrium.
From www.youtube.com
The electrolysis of copper (II) chloride. YouTube Copper Chloride Equilibrium This is known as le chatelier’s. Sample x was determined to be 0.15 m cucl 2 in h 2 o, while sample y was 0.15 m cucl 2 in 12 m hcl. To make the equilibrium mixture i give to students, i use some copper ii chloride, and add some sodium chloride or aluminum chloride to shift the equilibrium a. Copper Chloride Equilibrium.