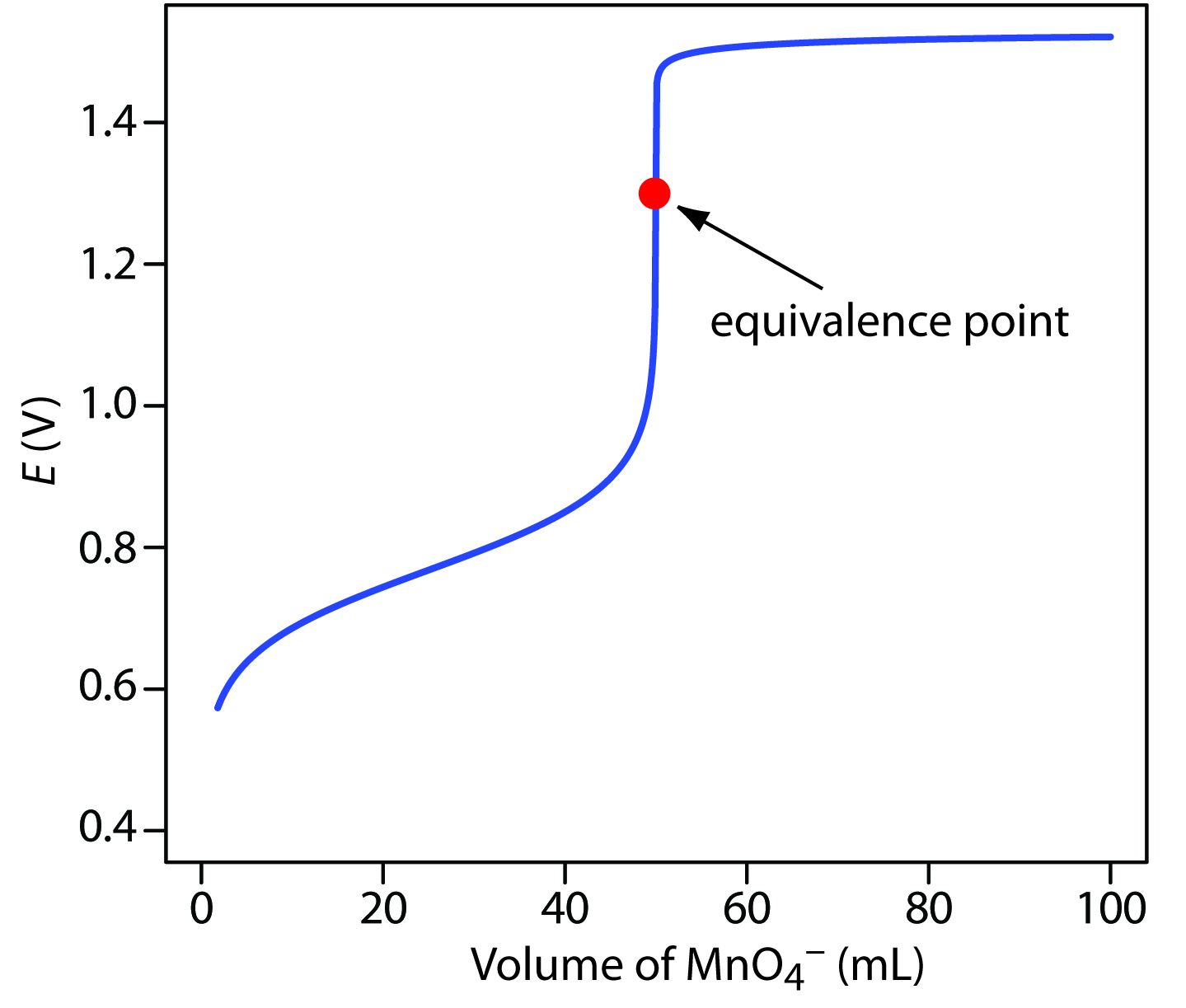
Equivalence Point For Titration . In an ideal world, the colour change would happen when you mix the two solutions together in the. The point at which this reaction is just complete is known as the equivalence point. In other words, while titrating, it. In a titration, it is. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. This is to be distinguished from the end point,. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. When the indicator changes colour, this is often described as the end point of the titration.
from chem.libretexts.org
In a titration, it is. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The point at which this reaction is just complete is known as the equivalence point. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. In other words, while titrating, it. This is to be distinguished from the end point,.
9.4 Redox Titrations Chemistry LibreTexts
Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. This is to be distinguished from the end point,. In an ideal world, the colour change would happen when you mix the two solutions together in the. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, it is. In other words, while titrating, it. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The point at which this reaction is just complete is known as the equivalence point. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When the indicator changes colour, this is often described as the end point of the titration.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. The point at which this reaction is just complete is known as the equivalence point. When the indicator changes colour, this is often described as the end point of the titration. In a. Equivalence Point For Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point For Titration The point at which this reaction is just complete is known as the equivalence point. In other words, while titrating, it. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have. Equivalence Point For Titration.
From www.youtube.com
Titrations and the M1V1=M2V2 Math at The Equivalence Point YouTube Equivalence Point For Titration This is to be distinguished from the end point,. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, it is. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize. Equivalence Point For Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Equivalence Point For Titration In other words, while titrating, it. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added titrant is. Equivalence Point For Titration.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Equivalence Point For Titration This is to be distinguished from the end point,. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The point at which this reaction is just complete is. Equivalence Point For Titration.
From www.chemistrystudent.com
Finding Ka using a Titration Curve (A2level) ChemistryStudent Equivalence Point For Titration In an ideal world, the colour change would happen when you mix the two solutions together in the. In other words, while titrating, it. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The higher molarity of the acid compared to the base in. Equivalence Point For Titration.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie Equivalence Point For Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In other words, while titrating, it. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point of a chemical reaction is the point at which equal. Equivalence Point For Titration.
From www.youtube.com
Titration curves in details. Equivalence point. Half equivalence point Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In other words, while titrating, it. In an ideal world, the. Equivalence Point For Titration.
From www.vrogue.co
How To Find Equivalence Point From Titration Data vrogue.co Equivalence Point For Titration This is to be distinguished from the end point,. The point at which this reaction is just complete is known as the equivalence point. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed. Equivalence Point For Titration.
From www.youtube.com
Strong acid / strong base titration pH at equivalence point YouTube Equivalence Point For Titration The point at which this reaction is just complete is known as the equivalence point. When the indicator changes colour, this is often described as the end point of the titration. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The higher molarity of the acid compared. Equivalence Point For Titration.
From mavink.com
What Is The Equivalence Point Of A Titration Equivalence Point For Titration In other words, while titrating, it. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. The point at which this reaction is just complete is known as the equivalence point. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly. Equivalence Point For Titration.
From www.differencebetween.com
Difference Between Half Equivalence Point and Equivalence Point Equivalence Point For Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. Equivalence Point For Titration.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Equivalence Point For Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. This is to be distinguished from the end point,. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In an ideal world, the colour change. Equivalence Point For Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Atoms First Equivalence Point For Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. The point at which this reaction is just complete is known as the equivalence point. This is to be distinguished from the end point,. In a titration, it is. The higher molarity of the acid. Equivalence Point For Titration.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Equivalence Point For Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The point at which this reaction is just complete is known as the equivalence point. The higher molarity of the acid compared to the base in this case means that a smaller volume of the. Equivalence Point For Titration.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Equivalence Point For Titration The point at which this reaction is just complete is known as the equivalence point. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The. Equivalence Point For Titration.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. The point at which this reaction is just complete is known as the equivalence point. In an ideal world, the colour change would happen when you mix the two solutions together in the.. Equivalence Point For Titration.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie Equivalence Point For Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In a titration, it is. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. In other words, while titrating, it. A point of. Equivalence Point For Titration.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Equivalence Point For Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. This is to be distinguished from the end point,. The higher molarity of the acid compared to the base. Equivalence Point For Titration.
From www.numerade.com
SOLVEDWhat is a titration? What is the equivalence point? Equivalence Point For Titration This is to be distinguished from the end point,. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. In other words, while titrating, it. In an ideal world, the colour change would happen when you mix the two solutions together in the. In a. Equivalence Point For Titration.
From www.showme.com
Titration Curve Explained Science, Chemistry ShowMe Equivalence Point For Titration When the indicator changes colour, this is often described as the end point of the titration. The point at which this reaction is just complete is known as the equivalence point. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. The equivalence. Equivalence Point For Titration.
From oneclass.com
CHE 2B Study Guide Spring 2019, Final Titration, Equivalence Point Equivalence Point For Titration The point at which this reaction is just complete is known as the equivalence point. In an ideal world, the colour change would happen when you mix the two solutions together in the. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. In a titration, it. Equivalence Point For Titration.
From www.youtube.com
Finding equivalence point for Monoprotic acid YouTube Equivalence Point For Titration The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When the indicator changes colour, this is often described as the end. Equivalence Point For Titration.
From mavink.com
Acid Base Titration Curve Equivalence Point For Titration In other words, while titrating, it. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. The equivalence. Equivalence Point For Titration.
From psiberg.com
The Equivalence Point Acid/Base Titrations PSIBERG Equivalence Point For Titration The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent quantities of reactants have been. In a titration, it is. In an ideal world, the colour change would happen when you mix the two solutions together in the. In other words, while titrating, it. A point of equivalence in a titration refers to. Equivalence Point For Titration.
From www.chegg.com
Solved Identify the first equivalence point on the titration Equivalence Point For Titration In a titration, it is. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. This is to be distinguished from the end point,. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize. Equivalence Point For Titration.
From www.youtube.com
Titration Curves, Equivalence Point YouTube Equivalence Point For Titration The point at which this reaction is just complete is known as the equivalence point. In a titration, it is. In other words, while titrating, it. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. A point of equivalence in a titration refers to a point at which the added. Equivalence Point For Titration.
From study.com
Finding the Equivalence Point Titration & Examples Lesson Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. In other words, while titrating, it. The point at which this reaction is just complete is known as the equivalence point. In an ideal world, the colour change would happen when you mix. Equivalence Point For Titration.
From www.bartleby.com
Answered Ca(OH)2 Titration Data for Ksp 12… bartleby Equivalence Point For Titration In other words, while titrating, it. In a titration, it is. The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. This is to be distinguished from the end point,. A point of equivalence in a titration refers to a point at which. Equivalence Point For Titration.
From www.youtube.com
How to Find the Equivalence Point on a Titration Graph In Excel YouTube Equivalence Point For Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. In other words, while titrating, it. When the indicator changes colour, this is often described as the end. Equivalence Point For Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Equivalence Point For Titration The higher molarity of the acid compared to the base in this case means that a smaller volume of the acid is required to reach the equivalence. This is to be distinguished from the end point,. In a titration, it is. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent. Equivalence Point For Titration.
From mungfali.com
Equivalence Points On Titration Graph Equivalence Point For Titration In other words, while titrating, it. A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. When the indicator changes colour, this. Equivalence Point For Titration.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Equivalence Point For Titration A point of equivalence in a titration refers to a point at which the added titrant is chemically equivalent to the sample analyte. When the indicator changes colour, this is often described as the end point of the titration. In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point. Equivalence Point For Titration.
From chemistrytalk.org
Titration Curves & Equivalence Point Calculations ChemTalk Equivalence Point For Titration The equivalence point of a chemical reaction is the point at which equal quantities of reactants are mixed chemically. This is to be distinguished from the end point,. The point at which this reaction is just complete is known as the equivalence point. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly. Equivalence Point For Titration.
From www.chegg.com
Solved Identify the equivalence point on the titration curve Equivalence Point For Titration In an ideal world, the colour change would happen when you mix the two solutions together in the. The equivalence point or stoichiometric point is the point in a chemical reaction when there is exactly enough acid and base to neutralize the solution. The equivalence point, or stoichiometric point, of a chemical reaction is the point at which chemically equivalent. Equivalence Point For Titration.