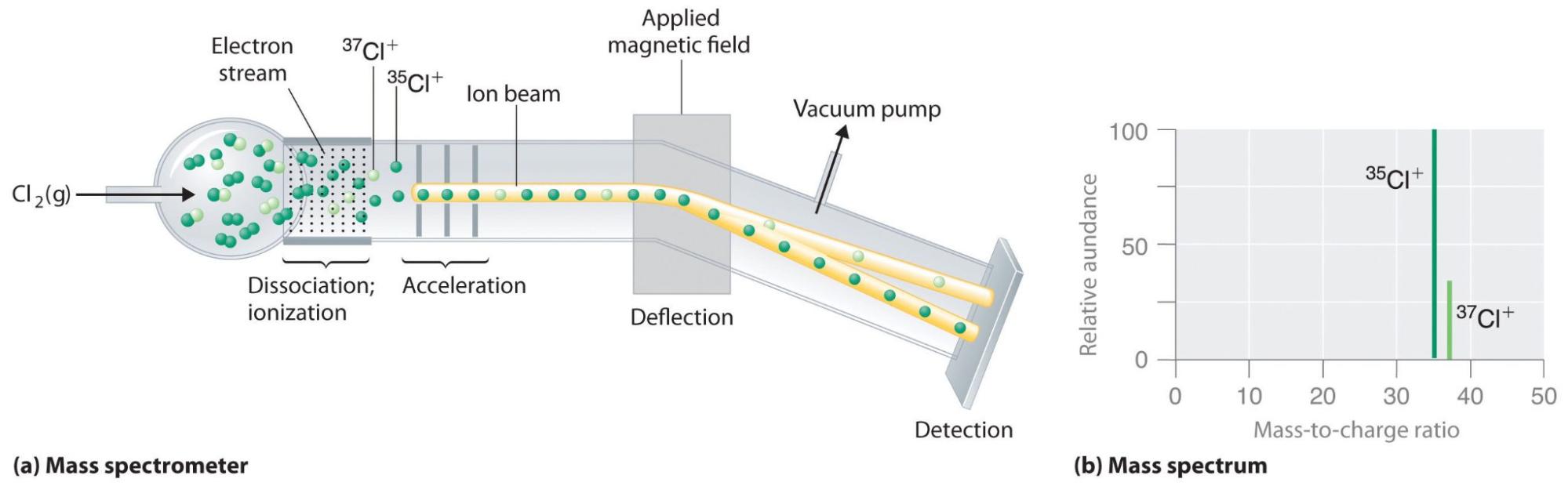
Chlorine Isotopes Abundance . The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The abundance of the isotopes. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; The relative atomic mass of an element can be. There are only two stable isotopes: In this case, you have the. The tallest peak is often given an arbitrary height of 100. 35 cl and 37 cl with. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. See examples of chlorine isotope patterns and how they differ. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope?
from alevelchemistry.co.uk
35 cl and 37 cl with. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. In this case, you have the. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? See examples of chlorine isotope patterns and how they differ. There are only two stable isotopes: The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The abundance of the isotopes.
Isotopic Abundance ALevel Chemistry Revision Notes
Chlorine Isotopes Abundance The relative atomic mass of an element can be. There are only two stable isotopes: The relative atomic mass of an element can be. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. The abundance of the isotopes. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; 35 cl and 37 cl with. See examples of chlorine isotope patterns and how they differ. The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The tallest peak is often given an arbitrary height of 100.
From www.chegg.com
Solved Fictitious element Z has three naturally occurring Chlorine Isotopes Abundance 35 cl and 37 cl with. In this case, you have the. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Because of this, the mass of an element is. Chlorine Isotopes Abundance.
From brainly.in
If chlorine atom is available in the form of two isotopes 35cl 75and Chlorine Isotopes Abundance The tallest peak is often given an arbitrary height of 100. 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are only two stable isotopes: Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the. Chlorine Isotopes Abundance.
From alevelchemistry.co.uk
Isotopic Abundance ALevel Chemistry Revision Notes Chlorine Isotopes Abundance The abundance of the isotopes. There are only two stable isotopes: The tallest peak is often given an arbitrary height of 100. See examples of chlorine isotope patterns and how they differ. The relative atomic mass of an element can be. Because of this, the mass of an element is given as relative atomic mass (a r) by using the. Chlorine Isotopes Abundance.
From byjus.com
7. Two isotopes of Boron have atomic mass 10 and 11.Calculate Chlorine Isotopes Abundance In this case, you have the. See examples of chlorine isotope patterns and how they differ. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; The relative atomic mass of an element can be. The relative sizes of the peaks gives you a direct measure. Chlorine Isotopes Abundance.
From www.showme.com
Relative abundancies of Chlorine from a mass spectrometer Science Chlorine Isotopes Abundance 35 cl and 37 cl with. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; See examples of chlorine isotope patterns and how they. Chlorine Isotopes Abundance.
From www.researchgate.net
Stable isotopes of chlorine Download Scientific Diagram Chlorine Isotopes Abundance The abundance of the isotopes. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. In this case, you have the. There are only two stable isotopes: Chlorine has two isotopes, with 75.53% being 35. Chlorine Isotopes Abundance.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Chlorine Isotopes Abundance In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. The relative sizes of the peaks gives you a. Chlorine Isotopes Abundance.
From www.vrogue.co
Education Information How To Calculate Average Atomic vrogue.co Chlorine Isotopes Abundance The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The tallest peak is often given an arbitrary height of 100. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? See examples of chlorine isotope patterns and. Chlorine Isotopes Abundance.
From byjus.com
cl 35 and cl 37 are two isotopes of chlorine . if average atomic mass Chlorine Isotopes Abundance See examples of chlorine isotope patterns and how they differ. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. 35 cl and 37 cl with. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other. Chlorine Isotopes Abundance.
From www.abhayjere.com
Calculating Average Atomic Mass Worksheet Chlorine Isotopes Abundance Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; 35 cl and 37 cl with. The tallest peak is often given an arbitrary height of 100. See examples of chlorine isotope patterns and how they differ. There are only two stable isotopes: The relative sizes. Chlorine Isotopes Abundance.
From www.slideserve.com
PPT KS4 Chemistry PowerPoint Presentation, free download ID5568233 Chlorine Isotopes Abundance The abundance of the isotopes. In this case, you have the. There are only two stable isotopes: The tallest peak is often given an arbitrary height of 100. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; Chlorine has 24 isotopes with mass numbers ranging. Chlorine Isotopes Abundance.
From askfilo.com
Chlorine has two isotopes 35Cl and 37Cl. If the abundance of lighter is.. Chlorine Isotopes Abundance The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The abundance of the isotopes. 35 cl and 37 cl with. The tallest peak is often given an. Chlorine Isotopes Abundance.
From pixels.com
Isotopes Of Chlorine Photograph by Photo Libary Chlorine Isotopes Abundance 35 cl and 37 cl with. See examples of chlorine isotope patterns and how they differ. The relative atomic mass of an element can be. There are only two stable isotopes: The abundance of the isotopes. The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The tallest peak is often given. Chlorine Isotopes Abundance.
From ar.inspiredpencil.com
Chlorine Isotope Chlorine Isotopes Abundance 35 cl and 37 cl with. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The tallest peak is often given an arbitrary height of 100. The relative atomic mass of an element can be. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average. Chlorine Isotopes Abundance.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Chlorine Isotopes Abundance Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The abundance of the isotopes. Because of this, the mass of an element is given as relative atomic mass (a r) by. Chlorine Isotopes Abundance.
From www.numerade.com
SOLVED Chlorine has two naturally occuring isotopes chlorine35 and Chlorine Isotopes Abundance Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? In this case, you have the. The tallest peak is often given an arbitrary height of 100. There are only two stable. Chlorine Isotopes Abundance.
From www.breakingatom.com
Isotopes of Elements Chlorine Isotopes Abundance In this case, you have the. In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. The tallest peak is often given an arbitrary height of 100. 35 cl and 37 cl with. Because of this, the mass of an element is given as relative atomic mass (a r). Chlorine Isotopes Abundance.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Abundance The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; There are only two stable isotopes: See examples of chlorine isotope patterns and how they differ. Chlorine has. Chlorine Isotopes Abundance.
From www.buyisotope.com
Chlorine37, Chlorine37 Isotope, Enriched Chlorine37 Chlorine Isotopes Abundance Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The abundance of the isotopes. There are only two stable isotopes: See examples of chlorine isotope patterns and how they differ. In this case, you have the. In the above, the most intense ion is set. Chlorine Isotopes Abundance.
From www.slideserve.com
PPT QUANTITTIES IN CHEMISTRY THE MOLE, MASS AND MOLAR MASS Chlorine Isotopes Abundance The abundance of the isotopes. In this case, you have the. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; In the above, the most intense ion is set to 100%. Chlorine Isotopes Abundance.
From byjus.com
60. If the average atomic mass of chlorine is 35.5, then find the Chlorine Isotopes Abundance Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. The tallest peak is often given an arbitrary height of 100. The relative atomic mass of an element. Chlorine Isotopes Abundance.
From www.numerade.com
Section A Answer all the questions in the spaces provided (a) Chlorine Chlorine Isotopes Abundance The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? There are only two stable isotopes: 35 cl and 37 cl with. The tallest peak is often given. Chlorine Isotopes Abundance.
From www.youtube.com
How to calculate percentage abundance of each isotope ? YouTube Chlorine Isotopes Abundance The tallest peak is often given an arbitrary height of 100. The abundance of the isotopes. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. 35 cl and 37 cl with.. Chlorine Isotopes Abundance.
From www.numerade.com
shilt option section a answer all the questions in the spaces provided Chlorine Isotopes Abundance The relative atomic mass of an element can be. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. In this case, you have the. The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Because of this, the mass of an element is given as relative atomic. Chlorine Isotopes Abundance.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Chlorine Isotopes Abundance There are only two stable isotopes: The tallest peak is often given an arbitrary height of 100. In this case, you have the. The abundance of the isotopes. The relative atomic mass of an element can be. The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. Chlorine has 24 isotopes with. Chlorine Isotopes Abundance.
From www.teachoo.com
Isotopes and Isobars Definition, Uses and Difference Teachoo Chlorine Isotopes Abundance The relative atomic mass of an element can be. There are only two stable isotopes: Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The abundance of the isotopes. See examples of chlorine isotope patterns and how they differ. The tallest peak is often given. Chlorine Isotopes Abundance.
From scienceprism.co.uk
How archaeologists use chemistry to find out about our past Science Prism Chlorine Isotopes Abundance In the above, the most intense ion is set to 100% since this corresponds best to the output from a mass. See examples of chlorine isotope patterns and how they differ. The tallest peak is often given an arbitrary height of 100. Because of this, the mass of an element is given as relative atomic mass (a r) by using. Chlorine Isotopes Abundance.
From periodictable.me
Way to Find Atomic Mass of Elements Dynamic Periodic Table of Chlorine Isotopes Abundance There are only two stable isotopes: In this case, you have the. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. See examples of chlorine isotope patterns and how they differ. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope?. Chlorine Isotopes Abundance.
From www.youtube.com
13.04 Isotopic Abundance in Mass Spectrometry YouTube Chlorine Isotopes Abundance In this case, you have the. See examples of chlorine isotope patterns and how they differ. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? 35 cl and 37 cl with. Because of this, the mass of an element is given as relative atomic mass. Chlorine Isotopes Abundance.
From www.coursehero.com
[Solved] The relative abundance of the two isotopes of chlorine are Chlorine Isotopes Abundance Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? The abundance of the isotopes. See examples of chlorine isotope patterns and how they differ. 35 cl and 37 cl with. The tallest peak is often given an arbitrary height of 100. The relative sizes of. Chlorine Isotopes Abundance.
From www.toppr.com
Chlorine has two naturally occurring isotopes, ^35Cl and ^37Cl . If the Chlorine Isotopes Abundance In this case, you have the. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. See examples of chlorine isotope patterns and how they differ. The relative atomic mass of an element can be. Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass. Chlorine Isotopes Abundance.
From lessonlibrarypaliform.z14.web.core.windows.net
How To Average Atomic Mass Chlorine Isotopes Abundance The relative atomic mass of an element can be. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? There are only two stable isotopes: Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass. Chlorine Isotopes Abundance.
From www.slideserve.com
PPT Mass Spectrometry (Mass Spec.) PowerPoint Presentation, free Chlorine Isotopes Abundance In this case, you have the. Chlorine has two isotopes, with 75.53% being 35 cl with an isotopic mass of 34.969 amu, what is the mass of the other isotope? Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. The relative atomic mass of an element can be. The relative sizes of the peaks gives. Chlorine Isotopes Abundance.
From www.coursehero.com
[Solved] Chlorine has two naturally occurring isotopes Chlorine 35 Chlorine Isotopes Abundance The relative sizes of the peaks gives you a direct measure of the relative abundances of the isotopes. See examples of chlorine isotope patterns and how they differ. Chlorine has 24 isotopes with mass numbers ranging from 28 cl to 51 cl. There are only two stable isotopes: The abundance of the isotopes. The tallest peak is often given an. Chlorine Isotopes Abundance.
From www.slideserve.com
PPT Relative atomic mass PowerPoint Presentation ID5933036 Chlorine Isotopes Abundance Because of this, the mass of an element is given as relative atomic mass (a r) by using the average mass of the isotopes; The tallest peak is often given an arbitrary height of 100. See examples of chlorine isotope patterns and how they differ. In this case, you have the. The relative atomic mass of an element can be.. Chlorine Isotopes Abundance.