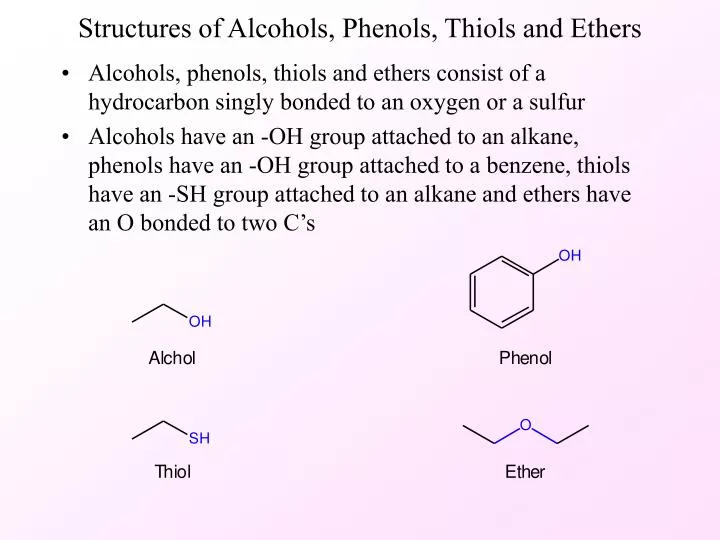
Difference Between Phenols And Alcohols . Phenols can be found as colourless solids present in the form of crystals. Both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Substituent groups on an aromatic ring that. Explain, in terms of inductive and resonance. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. The alcohols are a class of organic compounds that hold at least one hydroxyl. Mostly, alcohols are colorless and are in a liquid state. Explain why phenols are more acidic than alcohols. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base.
from www.slideserve.com
Phenols can be found as colourless solids present in the form of crystals. Explain, in terms of inductive and resonance. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Mostly, alcohols are colorless and are in a liquid state. Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. When deprotonated, their conjugate bases are both. Substituent groups on an aromatic ring that. Explain the difference in acidity between two given alcohols or phenols. Both alcohols and phenols are capable of acting as weakly acidic species;
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint
Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. The alcohols are a class of organic compounds that hold at least one hydroxyl. When deprotonated, their conjugate bases are both. Explain the difference in acidity between two given alcohols or phenols. Both alcohols and phenols are capable of acting as weakly acidic species; Explain, in terms of inductive and resonance. Substituent groups on an aromatic ring that. Explain why phenols are more acidic than alcohols. Phenols can be found as colourless solids present in the form of crystals. Mostly, alcohols are colorless and are in a liquid state.
From www.studypool.com
SOLUTION Chapter 11 alcohols phenols and ethers class 12 chemistry Difference Between Phenols And Alcohols Phenols can be found as colourless solids present in the form of crystals. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Explain the difference in acidity between two given alcohols or phenols. Both alcohols. Difference Between Phenols And Alcohols.
From byjus.com
Phenols are more acidic than alcohol. Explain why? Difference Between Phenols And Alcohols When deprotonated, their conjugate bases are both. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain the difference in acidity between two given alcohols or phenols. The alcohols are a class of organic compounds that hold at least one hydroxyl. Phenols can be found as colourless solids present in the form of crystals.. Difference Between Phenols And Alcohols.
From www.youtube.com
Difference Between Alcohol And Phenol?Class Series YouTube Difference Between Phenols And Alcohols Explain the difference in acidity between two given alcohols or phenols. Both alcohols and phenols are capable of acting as weakly acidic species; Explain, in terms of inductive and resonance. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. When deprotonated, their conjugate bases are both. Explain why phenols are. Difference Between Phenols And Alcohols.
From www.youtube.com
Difference Between Alcohol and Phenol, Chemistry Lecture Sabaq.pk Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Explain the difference in acidity between two given alcohols or phenols. Substituent groups on an aromatic ring that. Mostly, alcohols are colorless and are in a liquid state. Both alcohols and phenols are. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT ORGANIC CHEMISTRY PowerPoint Presentation ID233852 Difference Between Phenols And Alcohols Both alcohols and phenols are capable of acting as weakly acidic species; Phenols can be found as colourless solids present in the form of crystals. When deprotonated, their conjugate bases are both. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The alcohols are a class of organic compounds that hold at least one. Difference Between Phenols And Alcohols.
From www.differencebetween.com
Difference Between Alcohols and Phenols Compare the Difference Difference Between Phenols And Alcohols Explain the difference in acidity between two given alcohols or phenols. When deprotonated, their conjugate bases are both. Mostly, alcohols are colorless and are in a liquid state. The alcohols are a class of organic compounds that hold at least one hydroxyl. Phenols can be found as colourless solids present in the form of crystals. Explain why phenols are more. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Chapter 17 Alcohols and Phenols PowerPoint Presentation, free Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain, in terms of inductive and resonance. Substituent groups on an aromatic ring that. When deprotonated, their conjugate bases are both. The alcohols are a class of organic compounds that hold at least one hydroxyl. Mostly, alcohols are colorless and are in a liquid state.. Difference Between Phenols And Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Difference Between Phenols And Alcohols Phenols can be found as colourless solids present in the form of crystals. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Difference Between Phenols And Alcohols When deprotonated, their conjugate bases are both. Substituent groups on an aromatic ring that. Explain why phenols are more acidic than alcohols. Phenols can be found as colourless solids present in the form of crystals. Mostly, alcohols are colorless and are in a liquid state. Explain the difference in acidity between two given alcohols or phenols. Explain, in terms of. Difference Between Phenols And Alcohols.
From pediaa.com
Difference Between Alcohol and Phenol Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Mostly, alcohols are colorless and are in a liquid state. The alcohols are a class of organic compounds that hold at least one hydroxyl. Substituent groups on an aromatic ring that. Phenols can be found as colourless solids present in the form of crystals. Explain. Difference Between Phenols And Alcohols.
From www.slideshare.net
Alcohols, Phenols, and Ethers Difference Between Phenols And Alcohols Explain, in terms of inductive and resonance. Phenols can be found as colourless solids present in the form of crystals. Mostly, alcohols are colorless and are in a liquid state. Both alcohols and phenols are capable of acting as weakly acidic species; Explain why phenols are more acidic than alcohols. Substituent groups on an aromatic ring that. Delocalization of the. Difference Between Phenols And Alcohols.
From www.masternotes.in
Alcohols phenols and ethers class 12 notes Master Notes Difference Between Phenols And Alcohols Phenols can be found as colourless solids present in the form of crystals. The alcohols are a class of organic compounds that hold at least one hydroxyl. Explain why phenols are more acidic than alcohols. Both alcohols and phenols are capable of acting as weakly acidic species; Delocalization of the negative charge over the ortho and para positions of the. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Structures of Alcohols, Phenols, Thiols and Ethers PowerPoint Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. When deprotonated, their conjugate bases are both. Phenols can be found as colourless solids present in the form of crystals. Explain, in terms of inductive and resonance. The alcohols are a class of organic compounds that hold. Difference Between Phenols And Alcohols.
From www.geeksforgeeks.org
Nomenclature of Alcohols, Phenols and Ethers Rules and Examples Difference Between Phenols And Alcohols Both alcohols and phenols are capable of acting as weakly acidic species; The alcohols are a class of organic compounds that hold at least one hydroxyl. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Mostly, alcohols are colorless and are in a liquid state. When deprotonated, their conjugate bases. Difference Between Phenols And Alcohols.
From studylib.net
Alcohols, Phenols and Ethers Difference Between Phenols And Alcohols The alcohols are a class of organic compounds that hold at least one hydroxyl. Phenols can be found as colourless solids present in the form of crystals. Mostly, alcohols are colorless and are in a liquid state. Substituent groups on an aromatic ring that. Explain the difference in acidity between two given alcohols or phenols. Delocalization of the negative charge. Difference Between Phenols And Alcohols.
From pediaa.com
Difference Between Alcohol and Carboxylic Acid Definition, Different Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Mostly, alcohols are colorless and are in a liquid state. Substituent groups on an aromatic ring that. Phenols can be found as colourless solids present in the form of crystals. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in. Difference Between Phenols And Alcohols.
From www.youtube.com
Difference between phenols and alcohol hydroxy compounds cbse Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Substituent groups on an aromatic ring that. The alcohols are a class of organic compounds that hold at least one hydroxyl. Mostly, alcohols are colorless and are in a liquid state. Explain, in terms of inductive and resonance. Phenols can be found as colourless solids present in the form of crystals. Phenols. Difference Between Phenols And Alcohols.
From studylib.net
Alcohols and Phenols Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain, in terms of inductive and resonance. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Substituent groups on an aromatic ring that. Mostly, alcohols are colorless and are in a liquid state. The alcohols. Difference Between Phenols And Alcohols.
From www.youtube.com
Alcohols & Phenols Lec 7 Difference between Alcohol and Phenol Ali Difference Between Phenols And Alcohols Explain the difference in acidity between two given alcohols or phenols. Mostly, alcohols are colorless and are in a liquid state. Phenols can be found as colourless solids present in the form of crystals. The alcohols are a class of organic compounds that hold at least one hydroxyl. Delocalization of the negative charge over the ortho and para positions of. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT ALCOHOLS, PHENOLS and ETHERS PowerPoint Presentation, free Difference Between Phenols And Alcohols When deprotonated, their conjugate bases are both. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Explain why phenols are more acidic than alcohols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Both alcohols and phenols are capable of acting as weakly acidic. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Alcohols and Phenols PowerPoint Presentation, free download ID Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance. Mostly, alcohols are colorless and are in a liquid state. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Phenols can be found as colourless solids present in the form of crystals. Both alcohols and. Difference Between Phenols And Alcohols.
From www.differencebetween.com
Difference Between Alcohols and Phenols Compare the Difference Difference Between Phenols And Alcohols Phenols can be found as colourless solids present in the form of crystals. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Explain, in terms of inductive and resonance. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Explain why phenols are more acidic. Difference Between Phenols And Alcohols.
From www.slideshare.net
Alcohols, Phenols, and Ethers Difference Between Phenols And Alcohols Explain the difference in acidity between two given alcohols or phenols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Mostly, alcohols are colorless and are in a liquid state. Substituent groups on an aromatic ring that. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Organic Chemistry II Alcohols, Phenols, Thiols , Ethers, and Difference Between Phenols And Alcohols Both alcohols and phenols are capable of acting as weakly acidic species; Explain why phenols are more acidic than alcohols. Explain, in terms of inductive and resonance. Phenols can be found as colourless solids present in the form of crystals. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Mostly, alcohols are colorless and. Difference Between Phenols And Alcohols.
From askfilo.com
Q. 6. Write any four differences between phenols and alcohols. Filo Difference Between Phenols And Alcohols Mostly, alcohols are colorless and are in a liquid state. The alcohols are a class of organic compounds that hold at least one hydroxyl. When deprotonated, their conjugate bases are both. Both alcohols and phenols are capable of acting as weakly acidic species; Explain, in terms of inductive and resonance. Substituent groups on an aromatic ring that. Phenols can be. Difference Between Phenols And Alcohols.
From byjus.com
What is the order of acidity of alcohols and phenols? Explain both in Difference Between Phenols And Alcohols The alcohols are a class of organic compounds that hold at least one hydroxyl. Explain the difference in acidity between two given alcohols or phenols. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Both alcohols and phenols are capable of acting as weakly acidic species; Mostly, alcohols are colorless and are in a. Difference Between Phenols And Alcohols.
From www.pdfprof.com
alcohols phenols and ethers pdf notes Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols can be found as colourless solids present in the form of crystals. Both alcohols and phenols are capable of acting as weakly acidic species; Explain, in terms of inductive and resonance. Explain why phenols are more acidic than alcohols. Explain the difference in acidity. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT Classification and Identification of Alcohols and Phenols Difference Between Phenols And Alcohols Phenols can be found as colourless solids present in the form of crystals. Explain why phenols are more acidic than alcohols. When deprotonated, their conjugate bases are both. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. The alcohols are a class of organic compounds that hold at least one. Difference Between Phenols And Alcohols.
From pediaa.com
Difference Between Alcohol and Phenol Definition, Structure, Use Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. Phenols can be found as colourless solids present in the form of crystals. Explain, in terms of inductive and resonance. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. When deprotonated, their conjugate bases are. Difference Between Phenols And Alcohols.
From www.cleariitmedical.com
Alcohols, Phenols and Ethers Chemistry Notes for IITJEE/NEET Difference Between Phenols And Alcohols Both alcohols and phenols are capable of acting as weakly acidic species; Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. When deprotonated, their conjugate bases are both. Explain why phenols are more acidic than alcohols. Mostly, alcohols are colorless and are in a liquid state. Delocalization of the negative. Difference Between Phenols And Alcohols.
From www.slideserve.com
PPT ALCOHOLS, PHENOLS and ETHERS PowerPoint Presentation ID6799930 Difference Between Phenols And Alcohols Delocalization of the negative charge over the ortho and para positions of the aromatic ring. The alcohols are a class of organic compounds that hold at least one hydroxyl. Phenols can be found as colourless solids present in the form of crystals. When deprotonated, their conjugate bases are both. Explain why phenols are more acidic than alcohols. Both alcohols and. Difference Between Phenols And Alcohols.
From www.myxxgirl.com
Ncert Alcohols Phenols Ethers Class Chemistry Handwritten Notes My Difference Between Phenols And Alcohols Explain, in terms of inductive and resonance. Delocalization of the negative charge over the ortho and para positions of the aromatic ring. When deprotonated, their conjugate bases are both. Explain why phenols are more acidic than alcohols. Phenols are more acidic than alcohols due to resonance delocalization of the negative charge in the phenoxide conjugate base. Explain the difference in. Difference Between Phenols And Alcohols.
From www.numerade.com
SOLVED 1 Draw structures of one primary alcohol, one secondary alcohol Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Explain the difference in acidity between two given alcohols or phenols. Explain, in terms of inductive and resonance. Both alcohols and phenols are capable of acting as weakly acidic species; The alcohols are a class of organic compounds that hold at least one hydroxyl. Delocalization of the negative charge over the ortho. Difference Between Phenols And Alcohols.
From www.toppr.com
Alcohols, Phenols And Ethers Cheatsheets Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Mostly, alcohols are colorless and are in a liquid state. The alcohols are a class of organic compounds that hold at least one hydroxyl. Explain the difference in acidity between two given alcohols or phenols. Phenols can be found as colourless solids present in the form of crystals. Phenols are more acidic. Difference Between Phenols And Alcohols.
From sciencemotive.com
Solved Questions Alcohols, Phenols and Ethers ScienceMotive Difference Between Phenols And Alcohols Explain why phenols are more acidic than alcohols. Mostly, alcohols are colorless and are in a liquid state. The alcohols are a class of organic compounds that hold at least one hydroxyl. Substituent groups on an aromatic ring that. Both alcohols and phenols are capable of acting as weakly acidic species; Phenols can be found as colourless solids present in. Difference Between Phenols And Alcohols.