If Bromine Atom Gains An Electron It Becomes . For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Thus, typically, metals (with nearly. Tell students that when an atom gains or loses an electron, it becomes an ion. However, if atoms lose or gain electrons, they become charged particles called ions. When an atom loses an electron it gains a positive charge and is called a cation. When an atom gains an electron it gains a. Since it has 1 more. You can see how this happens in the. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative.
from www.bartleby.com
However, if atoms lose or gain electrons, they become charged particles called ions. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Thus, typically, metals (with nearly. When an atom loses an electron it gains a positive charge and is called a cation. You can see how this happens in the. When an atom gains an electron it gains a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Since it has 1 more. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons.
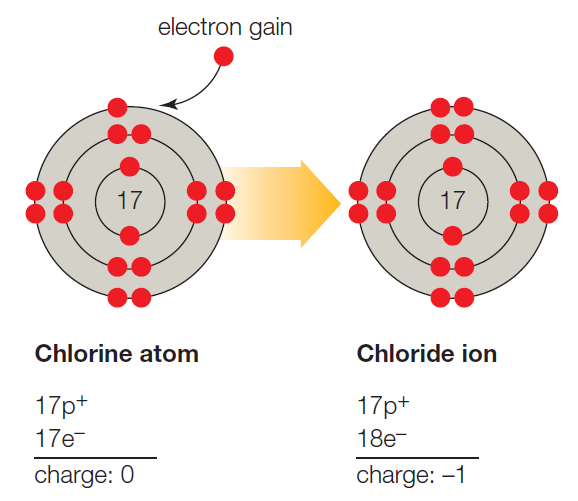
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl −
If Bromine Atom Gains An Electron It Becomes Since it has 1 more. You can see how this happens in the. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Since it has 1 more. When an atom gains an electron it gains a. Tell students that when an atom gains or loses an electron, it becomes an ion. However, if atoms lose or gain electrons, they become charged particles called ions. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Thus, typically, metals (with nearly. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. When an atom loses an electron it gains a positive charge and is called a cation.
From slideplayer.com
5.5 Atoms and Ions. ppt download If Bromine Atom Gains An Electron It Becomes You can see how this happens in the. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. When an atom gains an electron it gains a. Thus, typically, metals (with nearly. Since it has 1 more. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons.. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVED Successive ionization energy 4ca needed In this question When If Bromine Atom Gains An Electron It Becomes You can see how this happens in the. When an atom loses an electron it gains a positive charge and is called a cation. However, if atoms lose or gain electrons, they become charged particles called ions. Since it has 1 more. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a. If Bromine Atom Gains An Electron It Becomes.
From www.animalia-life.club
Electron Configuration For Bromine If Bromine Atom Gains An Electron It Becomes However, if atoms lose or gain electrons, they become charged particles called ions. When an atom gains an electron it gains a. Tell students that when an atom gains or loses an electron, it becomes an ion. Thus, typically, metals (with nearly. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron. If Bromine Atom Gains An Electron It Becomes.
From www.bartleby.com
B. A chlorine atom (Cl) a negatively charged chloride ion (Cl − If Bromine Atom Gains An Electron It Becomes Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. When an atom loses an electron it gains a positive charge and is called a cation. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. However, if. If Bromine Atom Gains An Electron It Becomes.
From www.ck12.org
to CK12 Foundation CK12 Foundation If Bromine Atom Gains An Electron It Becomes When an atom gains an electron it gains a. You can see how this happens in the. Thus, typically, metals (with nearly. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to. If Bromine Atom Gains An Electron It Becomes.
From dxopaibhs.blob.core.windows.net
A Chlorine Atom Gains An Electron. What Is The Resulting Particle at If Bromine Atom Gains An Electron It Becomes Atoms and chemical species lose or gain electrons when they react in order to gain stability. However, if atoms lose or gain electrons, they become charged particles called ions. When an atom gains an electron it gains a. You can see how this happens in the. An atom of bromine in the gas phase, for example, gives off energy when. If Bromine Atom Gains An Electron It Becomes.
From www.doubtnut.com
The electron affinity of bromine atom is equal to the..........of brom If Bromine Atom Gains An Electron It Becomes When an atom gains an electron it gains a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms and chemical species lose or gain electrons when they react in order to gain stability. An atom of bromine in the gas phase, for example, gives off energy when. If Bromine Atom Gains An Electron It Becomes.
From www.nagwa.com
Question Video Recalling the Species Formed When a Chlorine Atom Gains If Bromine Atom Gains An Electron It Becomes You can see how this happens in the. Tell students that when an atom gains or loses an electron, it becomes an ion. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Atoms and chemical species lose or gain electrons when they react in order. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVEDWhat is the electron configuration of a bromine atom (You CAN If Bromine Atom Gains An Electron It Becomes Tell students that when an atom gains or loses an electron, it becomes an ion. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. When an atom gains an electron it gains a. Ions form when atoms lose or gain. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVED Part E Write symbol without electron dots, for the ion formed If Bromine Atom Gains An Electron It Becomes Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. When an atom gains an electron it gains a. Thus, typically, metals (with nearly. You can see how this happens in the. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons. If Bromine Atom Gains An Electron It Becomes.
From www.slideserve.com
PPT Chapter 22 Chemical Bonds PowerPoint Presentation, free If Bromine Atom Gains An Electron It Becomes However, if atoms lose or gain electrons, they become charged particles called ions. Since it has 1 more. Atoms and chemical species lose or gain electrons when they react in order to gain stability. You can see how this happens in the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide. If Bromine Atom Gains An Electron It Becomes.
From www.coursehero.com
[Solved] 7. Explain What happens to an atom's charges when it gains or If Bromine Atom Gains An Electron It Becomes Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Atoms and chemical species lose or gain electrons when they react in order to gain stability. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Since it has 1 more. When an atom gains. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Chp. 2 Atoms and the Periodic Table ppt download If Bromine Atom Gains An Electron It Becomes Since it has 1 more. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Ions form when atoms lose or gain electrons close electron subatomic. If Bromine Atom Gains An Electron It Becomes.
From www.bartleby.com
Answered 1. When an atom gains 2 electrons, it… bartleby If Bromine Atom Gains An Electron It Becomes When an atom gains an electron it gains a. Thus, typically, metals (with nearly. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. You can see how this happens in the. Since it has 1 more. Tell students that when an atom gains or loses. If Bromine Atom Gains An Electron It Becomes.
From klaehyagl.blob.core.windows.net
Bromine Molecule Formula at Antonio Godines blog If Bromine Atom Gains An Electron It Becomes When an atom loses an electron it gains a positive charge and is called a cation. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Tell students that when an atom gains or loses an electron, it becomes an ion. When an atom gains an electron it. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Ionic and Covalent Bonds ppt download If Bromine Atom Gains An Electron It Becomes Thus, typically, metals (with nearly. You can see how this happens in the. Tell students that when an atom gains or loses an electron, it becomes an ion. However, if atoms lose or gain electrons, they become charged particles called ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. If Bromine Atom Gains An Electron It Becomes.
From jazlyndesnhwu.blogspot.com
Excited State Electron Configuration of Bromine If Bromine Atom Gains An Electron It Becomes You can see how this happens in the. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Thus, typically, metals (with nearly. Ions form when atoms lose or gain electrons close electron. If Bromine Atom Gains An Electron It Becomes.
From physicalsciencetext.weebly.com
7.6 Ions Physical Science If Bromine Atom Gains An Electron It Becomes Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Tell students that when an atom gains or loses an electron, it becomes an ion. Atoms and chemical species lose or gain electrons when they react in order to gain stability. When an atom loses an electron it gains. If Bromine Atom Gains An Electron It Becomes.
From brainly.ph
1. Is the number of proton and electron the same in Chlorine atom?2 If Bromine Atom Gains An Electron It Becomes When an atom gains an electron it gains a. You can see how this happens in the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. When an atom loses an electron it gains. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVED WRITE THE NAME AND SYMBOL OF THE ION FORMED WHEN 1. A CHLORINE If Bromine Atom Gains An Electron It Becomes Tell students that when an atom gains or loses an electron, it becomes an ion. However, if atoms lose or gain electrons, they become charged particles called ions. You can see how this happens in the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Sodium loses. If Bromine Atom Gains An Electron It Becomes.
From exydxgcqz.blob.core.windows.net
What Ionic Charge Does Bromine Have at Donna Wiley blog If Bromine Atom Gains An Electron It Becomes Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it. If Bromine Atom Gains An Electron It Becomes.
From www.slideserve.com
PPT Ions PowerPoint Presentation, free download ID2521245 If Bromine Atom Gains An Electron It Becomes Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. You can see how this happens in the. An atom of bromine in the gas phase, for example, gives. If Bromine Atom Gains An Electron It Becomes.
From www.nagwa.com
Question Video Determining What Forms When a Sodium Atom Loses One If Bromine Atom Gains An Electron It Becomes When an atom loses an electron it gains a positive charge and is called a cation. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Tell students that when an atom gains or loses an electron,. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVED What is the valence electron configuration for the bromine atom? If Bromine Atom Gains An Electron It Becomes However, if atoms lose or gain electrons, they become charged particles called ions. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Thus, typically, metals (with. If Bromine Atom Gains An Electron It Becomes.
From www.animalia-life.club
Electron Configuration For Bromine If Bromine Atom Gains An Electron It Becomes Atoms and chemical species lose or gain electrons when they react in order to gain stability. When an atom gains an electron it gains a. However, if atoms lose or gain electrons, they become charged particles called ions. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Thus, typically,. If Bromine Atom Gains An Electron It Becomes.
From www.nuclear-power.com
Bromine Electron Affinity Electronegativity Ionization Energy of If Bromine Atom Gains An Electron It Becomes Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Thus, typically, metals (with nearly. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. When an atom loses an electron it gains a positive charge and is called a cation. However, if atoms lose or. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Chapter 4 Formation of Compounds ppt download If Bromine Atom Gains An Electron It Becomes When an atom gains an electron it gains a. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. However, if atoms lose or gain electrons, they become charged particles called ions. When an atom loses an electron it gains a positive charge and is called a cation. Since. If Bromine Atom Gains An Electron It Becomes.
From cemauyoj.blob.core.windows.net
Highest Binding Energy Of An Atom Is at Byron Folden blog If Bromine Atom Gains An Electron It Becomes Thus, typically, metals (with nearly. Since it has 1 more. You can see how this happens in the. Atoms and chemical species lose or gain electrons when they react in order to gain stability. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. When an atom gains. If Bromine Atom Gains An Electron It Becomes.
From www.chegg.com
Solved Select all the true statements. When an atom gains If Bromine Atom Gains An Electron It Becomes Since it has 1 more. When an atom loses an electron it gains a positive charge and is called a cation. Atoms and chemical species lose or gain electrons when they react in order to gain stability. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Thus, typically, metals (with nearly. Ions form when atoms lose. If Bromine Atom Gains An Electron It Becomes.
From www.gauthmath.com
Solved Which particle represents the size of the bromide ion compared If Bromine Atom Gains An Electron It Becomes However, if atoms lose or gain electrons, they become charged particles called ions. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Since it has 1 more. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Atoms and chemical species lose or gain electrons. If Bromine Atom Gains An Electron It Becomes.
From basichemistry.blogspot.com
Basic Chemistry October 2012 If Bromine Atom Gains An Electron It Becomes However, if atoms lose or gain electrons, they become charged particles called ions. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Sodium loses an electron,. If Bromine Atom Gains An Electron It Becomes.
From www.numerade.com
SOLVEDUse orbital diagrams to illustrate what happens when an oxygen If Bromine Atom Gains An Electron It Becomes Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. For example, the neutral bromine atom, with 35 protons and 35 electrons, can gain one electron to provide it with 36 electrons. Atoms and chemical species. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Unit 1 Atomic Structure ppt download If Bromine Atom Gains An Electron It Becomes An atom of bromine in the gas phase, for example, gives off energy when it gains an electron to form an ion of bromine. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. You can see. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Structure of the Atom Atom smallest particle of an element that has If Bromine Atom Gains An Electron It Becomes When an atom loses an electron it gains a positive charge and is called a cation. When an atom gains an electron it gains a. Tell students that when an atom gains or loses an electron, it becomes an ion. Sodium loses an electron, leaving it with 11 protons, but only 10 electrons. Thus, typically, metals (with nearly. You can. If Bromine Atom Gains An Electron It Becomes.
From slideplayer.com
Chapter 4 Formation of Compounds ppt download If Bromine Atom Gains An Electron It Becomes Atoms that lose electrons acquire a positive charge because they are left with fewer negatively charged electrons to balance the. Ions form when atoms lose or gain electrons close electron subatomic particle, with a negative charge and a negligible mass relative. When an atom gains an electron it gains a. When an atom loses an electron it gains a positive. If Bromine Atom Gains An Electron It Becomes.