Osmometry Number Average Molecular Weight . Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for measuring mn are.
from www.numerade.com
We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for measuring mn are. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution.
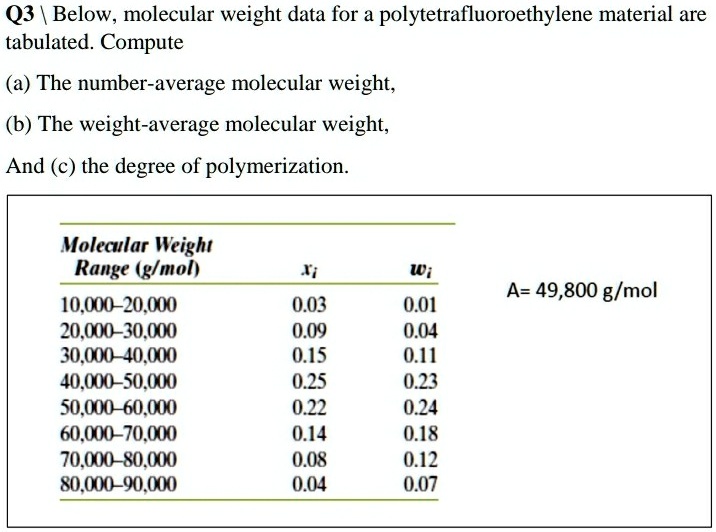
SOLVED Q3 Below, molecular weight data for a polytetrafluoroethylene
Osmometry Number Average Molecular Weight We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Most common methods for measuring mn are. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers:
From www.researchgate.net
Numberaverage molecular weight (M n,GPC ) and molecular weight Osmometry Number Average Molecular Weight We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Number average of molecular weight (m n) number average of molecular weight is measured to determine number. Osmometry Number Average Molecular Weight.
From www.researchgate.net
Number average molecular weight ( ) vs. conversion plot for ROMP of Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Most common methods for measuring mn are. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Number average of molecular weight (m n) number average of molecular weight. Osmometry Number Average Molecular Weight.
From www.youtube.com
Calculate Molecular Weight from Osmotic Pressure for Nonideal Solution Osmometry Number Average Molecular Weight Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number. Osmometry Number Average Molecular Weight.
From www.youtube.com
MSE 201 S21 Lecture 29 Module 1 Polymer Molecular Weight YouTube Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number.. Osmometry Number Average Molecular Weight.
From oneclass.com
OneClass Molecular weight data 1or polyethylene are shown below. (a Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer. Osmometry Number Average Molecular Weight.
From www.numerade.com
SOLVED Fill in the table Molecular weight Number of chains 500010000 Osmometry Number Average Molecular Weight We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Most common methods for. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved The definition of average molecular weight is the Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the. Osmometry Number Average Molecular Weight.
From studylib.net
Membrane Osmometry ( , A , χ) M 2 Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. A relative technique used to determine the number average molecular weight (mn) of a polymer in a. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved Calculate the weight average molecular weight, Mw, of Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Most common methods for measuring mn are. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a. Osmometry Number Average Molecular Weight.
From www.numerade.com
SOLVED Q3 Below, molecular weight data for a polytetrafluoroethylene Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Most common methods for measuring mn are. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a. Osmometry Number Average Molecular Weight.
From www.bartleby.com
Answered The following table lists molecular… bartleby Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for measuring mn are. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which. Osmometry Number Average Molecular Weight.
From www.numerade.com
Using the following osmometry data at 37°C, find 1. The number Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Most common methods for measuring mn are. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. There are two principal methods of osmometry that are. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved The numberaverage molecular weight of a Osmometry Number Average Molecular Weight Most common methods for measuring mn are. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Number average of molecular weight (m n) number average. Osmometry Number Average Molecular Weight.
From byjus.com
Calculate the average molecular weight of the mixture of 25 Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Number average of molecular. Osmometry Number Average Molecular Weight.
From www.semanticscholar.org
Figure 1 from Characterization of Linear Polyethylene SRM The IX Osmometry Number Average Molecular Weight Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Most common methods for measuring mn are. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. There are two principal methods of osmometry that are suitable for determining average molecular weights. Osmometry Number Average Molecular Weight.
From www.differencebetween.com
Difference Between Number Average and Weight Average Molecular Weight Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Most common methods for measuring mn are. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. We will discuss methods based on colligative properties, which. Osmometry Number Average Molecular Weight.
From www.researchgate.net
plot (a) and evolution of numberaverage molecular weight (M Osmometry Number Average Molecular Weight Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for. Osmometry Number Average Molecular Weight.
From brainly.in
Explain number average molecular weight of polymer Brainly.in Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Part 1. Introduction to Polymer Science PowerPoint Presentation Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Most common methods for measuring. Osmometry Number Average Molecular Weight.
From nanohub.org
Courses nanoHUBU Physics of Electronic Polymers Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Determination of Molecular Weight and Molecular Weight Osmometry Number Average Molecular Weight Most common methods for measuring mn are. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. A relative technique used to determine the number average molecular weight (mn) of a polymer in a. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved Calculate the numberaverage molecular weight of a Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Most common methods for measuring. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Chapter 2. Molecular Weight and Polymer Solutions PowerPoint Osmometry Number Average Molecular Weight Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n. Osmometry Number Average Molecular Weight.
From www.researchgate.net
Numberaverage molecular weight (M n,GPC ) and molecular weight Osmometry Number Average Molecular Weight Most common methods for measuring mn are. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. Number average of molecular weight (m n) number average. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT General Concepts Chapter 2 PowerPoint Presentation, free download Osmometry Number Average Molecular Weight Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles.. Osmometry Number Average Molecular Weight.
From slideplayer.com
Summary Last week Different conformations and configurations of Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for measuring mn are. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which. Osmometry Number Average Molecular Weight.
From www.researchgate.net
Developments of monomer conversion and number average molecular weight Osmometry Number Average Molecular Weight We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Weight and number average molecular masses PowerPoint Osmometry Number Average Molecular Weight Most common methods for measuring mn are. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. There are two principal methods of osmometry that are. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved 9. Molecular weight data for some polymer are Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods. Osmometry Number Average Molecular Weight.
From www.researchgate.net
Numberaverage molecular weight (M n ), weightaverage molecular weight Osmometry Number Average Molecular Weight Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Most common methods for measuring mn are. There are two principal methods of osmometry that are suitable for determining average molecular weights. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Biomass Fundamentals Module 6 Fundamental Principles of Polymer Osmometry Number Average Molecular Weight We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. Membrane osmometry ( m. Osmometry Number Average Molecular Weight.
From www.slideserve.com
PPT Chapter 2. Molecular Weight and Polymer Solutions PowerPoint Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Most common methods for measuring. Osmometry Number Average Molecular Weight.
From www.chegg.com
Solved 5. Osmometry (see figure below) is an important Osmometry Number Average Molecular Weight Most common methods for measuring mn are. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the number of solute. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Number average of molecular weight (m n) number average of. Osmometry Number Average Molecular Weight.
From www.studocu.com
Molecular weight of polymers HW 9 Problem 14. a. To Find (a) The Osmometry Number Average Molecular Weight A relative technique used to determine the number average molecular weight (mn) of a polymer in a dilute polymer solution. We will discuss methods based on colligative properties, which depend on the number of molecules present and yield number. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which depends only on the. Osmometry Number Average Molecular Weight.
From www.youtube.com
Introduction to Polymers Lecture 4.2. Number average molecular Osmometry Number Average Molecular Weight There are two principal methods of osmometry that are suitable for determining average molecular weights of polymers: Most common methods for measuring mn are. Number average of molecular weight (m n) number average of molecular weight is measured to determine number of particles. Membrane osmometry ( m n , a 2, χ) osmotic pressure, π, is a colligative property which. Osmometry Number Average Molecular Weight.