Silver Mirror Reaction Formula . An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: Tollen’s test (silver mirror) description: An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. This qualitative lab test is also referred to as the silver mirror test. It uses the fact that aldehydes can be more easily oxidized than ketones. Glucose gives a positive result,. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Tollens test is a very useful method to distinguish between aldehydes and ketones. It exploits the fact that aldehydes are readily oxidized,.
from www.teachoo.com
Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: It exploits the fact that aldehydes are readily oxidized,. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. It uses the fact that aldehydes can be more easily oxidized than ketones. Tollen’s test (silver mirror) description: Glucose gives a positive result,. In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. This qualitative lab test is also referred to as the silver mirror test.
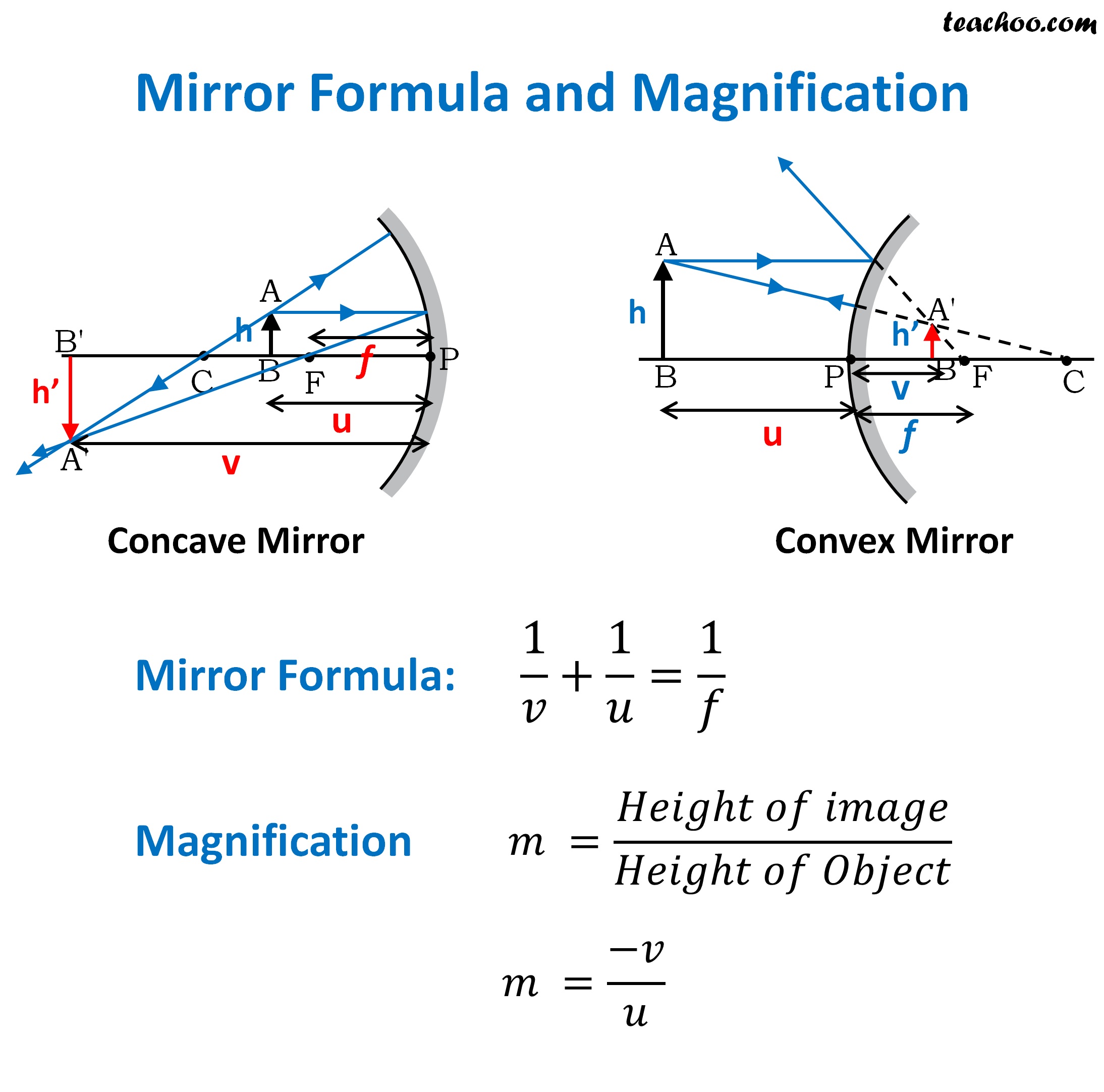
Mirror Formula with Solved Numericals Class 10 Teachoo
Silver Mirror Reaction Formula It uses the fact that aldehydes can be more easily oxidized than ketones. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. It exploits the fact that aldehydes are readily oxidized,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. It uses the fact that aldehydes can be more easily oxidized than ketones. Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: This qualitative lab test is also referred to as the silver mirror test. In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Glucose gives a positive result,. Tollen’s test (silver mirror) description: Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose.
From www.alamy.com
Silver mirror test with Tollens' reagent. 1 of 3 When a few drops of an Silver Mirror Reaction Formula Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Glucose gives a positive result,. Tollen’s test (silver mirror) description: It exploits the fact that aldehydes are readily oxidized,. An aldehyde. Silver Mirror Reaction Formula.
From docslib.org
Detection of Aldehydes Using Silver Mirror Reaction DocsLib Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. It exploits the fact that aldehydes are readily oxidized,. Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde group formula [wikipedia] for example, due to this process, we can find. Silver Mirror Reaction Formula.
From www.reddit.com
Copper and silver mirror reaction it turned out pretty well r/chemistry Silver Mirror Reaction Formula Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. It exploits the fact that aldehydes are readily oxidized,. It uses the fact that aldehydes can be more easily oxidized than ketones. Tollen's test for aldehydes and ketones (the silver. Silver Mirror Reaction Formula.
From leah4sci.com
Tollens Reagent Silver Mirror Test for Aldehydes Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollens test is a very useful method to distinguish between aldehydes and ketones. It uses the fact. Silver Mirror Reaction Formula.
From www.sciencephoto.com
Silver mirror test Stock Image C029/1101 Science Photo Library Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Tollens test is a very useful method to distinguish between aldehydes and ketones. It uses the fact that aldehydes can be more easily oxidized than ketones. Show your students how to make a mirror inside a. Silver Mirror Reaction Formula.
From fineartamerica.com
Silver Mirror Test Photograph by Andrew Lambert Photography Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by. Silver Mirror Reaction Formula.
From www.synarchive.com
Tollens Oxidation Silver Mirror Reaction Formula Glucose gives a positive result,. Tollens test is a very useful method to distinguish between aldehydes and ketones. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. It uses the fact that aldehydes can be more easily oxidized than ketones. An aldehyde group formula [wikipedia] for example,. Silver Mirror Reaction Formula.
From www.sciencephoto.com
Silver mirror test with Tollens' reagent Stock Image C029/1075 Silver Mirror Reaction Formula An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Glucose gives a positive result,. It uses the fact that aldehydes can be more easily oxidized than ketones. An aldehyde is. Silver Mirror Reaction Formula.
From www.teachoo.com
Mirror Formula with Solved Numericals Class 10 Teachoo Silver Mirror Reaction Formula Tollens test is a very useful method to distinguish between aldehydes and ketones. This qualitative lab test is also referred to as the silver mirror test. In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Tollen's test for aldehydes and ketones (the silver mirror) silver. Silver Mirror Reaction Formula.
From www.thesciencehive.co.uk
Redox and Electrode Potentials — the science hive Silver Mirror Reaction Formula An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Tollen’s test (silver mirror) description: This qualitative lab test is also referred to as the silver mirror test. Glucose gives a positive result,. An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains:. Silver Mirror Reaction Formula.
From www.sciencephoto.com
Silver mirror test Stock Image A500/0535 Science Photo Library Silver Mirror Reaction Formula An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which. Silver Mirror Reaction Formula.
From www.semanticscholar.org
Figure 1 from Siteselective deposition of silver nanoparticles using Silver Mirror Reaction Formula It exploits the fact that aldehydes are readily oxidized,. It uses the fact that aldehydes can be more easily oxidized than ketones. This qualitative lab test is also referred to as the silver mirror test. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Tollen’s test (silver mirror) description: An aldehyde group. Silver Mirror Reaction Formula.
From askfilo.com
Mirror Formula Simple Relation between the distance u of the object from.. Silver Mirror Reaction Formula An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: It exploits the fact that aldehydes are readily oxidized,. Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Show your students how to make a mirror inside. Silver Mirror Reaction Formula.
From byjus.com
A compound is heated with zinc dust and ammonium chloride followed by Silver Mirror Reaction Formula Tollen’s test (silver mirror) description: Tollens test is a very useful method to distinguish between aldehydes and ketones. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal,. Silver Mirror Reaction Formula.
From www.youtube.com
Tollens Test Silver mirror Reaction YouTube Silver Mirror Reaction Formula An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. Tollen’s test (silver mirror) description: It exploits the fact that aldehydes are readily oxidized,. Glucose gives a positive result,. Tollens test. Silver Mirror Reaction Formula.
From www.numerade.com
SOLVED 1. A compound of molecular formula C;HO forms yellow Silver Mirror Reaction Formula Tollens test is a very useful method to distinguish between aldehydes and ketones. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. Glucose gives a positive result,. Tollen’s test (silver mirror) description: In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent). Silver Mirror Reaction Formula.
From www.numerade.com
SOLVED Write and balance the chemical reaction for the formation of Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution. Silver Mirror Reaction Formula.
From byjus.com
Prove the mirror formula and explain it. Silver Mirror Reaction Formula It uses the fact that aldehydes can be more easily oxidized than ketones. This qualitative lab test is also referred to as the silver mirror test. Tollen’s test (silver mirror) description: In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Tollens test is a very. Silver Mirror Reaction Formula.
From fineartamerica.com
Silver Mirror Test Photograph by Fine Art America Silver Mirror Reaction Formula Glucose gives a positive result,. It exploits the fact that aldehydes are readily oxidized,. This qualitative lab test is also referred to as the silver mirror test. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollens test is a very useful method to distinguish between aldehydes and ketones. Tollen’s. Silver Mirror Reaction Formula.
From www.researchgate.net
(a) Closeup of the capillary without and with the inside silver film Silver Mirror Reaction Formula Tollen’s test (silver mirror) description: In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. This qualitative lab test is also referred to as the silver mirror test. Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and. Silver Mirror Reaction Formula.
From pubs.acs.org
Na2CO3 Mediated Silver Mirror Reaction of General Aldehyde at Room Silver Mirror Reaction Formula Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. This qualitative lab test is also referred to as the silver mirror test. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollen’s test (silver mirror) description: Tollen's. Silver Mirror Reaction Formula.
From fphoto.photoshelter.com
55360210F2RM Fundamental Photographs The Art of Science Silver Mirror Reaction Formula Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. This qualitative lab test is also referred to as the silver mirror test. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. In this experiment, students observe what happens when. Silver Mirror Reaction Formula.
From www.slideserve.com
PPT Revision formation of aldehydes and ketones from alcohols Silver Mirror Reaction Formula An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Show your students how to make a mirror inside a. Silver Mirror Reaction Formula.
From melscience.com
The silver mirror reaction MEL Chemistry Silver Mirror Reaction Formula This qualitative lab test is also referred to as the silver mirror test. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollens test is a very useful. Silver Mirror Reaction Formula.
From ar.inspiredpencil.com
Tollens Reagent Silver Mirror Reaction Formula An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Glucose gives a positive result,. Tollen’s test (silver mirror) description: It uses the fact that aldehydes. Silver Mirror Reaction Formula.
From www.vedantu.com
When liquid ‘A’ is treated with a freshly prepared ammoniacal silver Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. It uses the fact that aldehydes can be more easily oxidized than ketones. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. An aldehyde group formula [wikipedia] for. Silver Mirror Reaction Formula.
From theedge.com.hk
The Silver Mirror Test The Edge Silver Mirror Reaction Formula This qualitative lab test is also referred to as the silver mirror test. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Tollen’s test (silver mirror) description: Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Tollen's. Silver Mirror Reaction Formula.
From www.compoundchem.com
Making silver mirrors using chemistry Compound Interest Silver Mirror Reaction Formula Tollen’s test (silver mirror) description: It exploits the fact that aldehydes are readily oxidized,. It uses the fact that aldehydes can be more easily oxidized than ketones. This qualitative lab test is also referred to as the silver mirror test. Glucose gives a positive result,. Tollens test is a very useful method to distinguish between aldehydes and ketones. In this. Silver Mirror Reaction Formula.
From journals.sagepub.com
Preparation and Characterization of Silver Liquid Thin Films for Silver Mirror Reaction Formula It exploits the fact that aldehydes are readily oxidized,. Tollen's test for aldehydes and ketones (the silver mirror) silver (i) is reduced to silver metal by aldehydes, but not by ketones. Tollens test is a very useful method to distinguish between aldehydes and ketones. Glucose gives a positive result,. It uses the fact that aldehydes can be more easily oxidized. Silver Mirror Reaction Formula.
From www.youtube.com
Silver mirror reaction YouTube Silver Mirror Reaction Formula This qualitative lab test is also referred to as the silver mirror test. Glucose gives a positive result,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde group formula [wikipedia] for example, due to this process, we. Silver Mirror Reaction Formula.
From www.chegg.com
Solved The Tollen's test is the reaction of aldehydes with Silver Mirror Reaction Formula Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose. Glucose gives a positive result,. This qualitative lab test is also referred to as the silver mirror test. It exploits the fact that aldehydes are readily oxidized,. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver. Silver Mirror Reaction Formula.
From melscience.com
The silver mirror reaction MEL Chemistry Silver Mirror Reaction Formula An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. Show your students how to make a mirror inside a flask, using the reaction between silver nitrate and glucose.. Silver Mirror Reaction Formula.
From www.doubtnut.com
Explain silver mirror Test. Silver Mirror Reaction Formula Tollens test is a very useful method to distinguish between aldehydes and ketones. An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: Tollen’s test (silver mirror) description: An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal, which coats. This qualitative lab test is. Silver Mirror Reaction Formula.
From onlinesciencenotes.com
Tollen’s test or Silver mirror test Principle, Requirements, Procedure Silver Mirror Reaction Formula Glucose gives a positive result,. It exploits the fact that aldehydes are readily oxidized,. Tollens test is a very useful method to distinguish between aldehydes and ketones. Tollen’s test (silver mirror) description: This qualitative lab test is also referred to as the silver mirror test. An aldehyde is oxidized by silver (i) to generate a carboxylic acid and silver metal,. Silver Mirror Reaction Formula.
From www.tandfonline.com
Synthesis of silver nanofiber transparent electrodes by silver mirror Silver Mirror Reaction Formula In this experiment, students observe what happens when a solution containing silver nitrate (tollens’ reagent) and a reducing sugar (glucose) react to form silver. It exploits the fact that aldehydes are readily oxidized,. Tollen’s test (silver mirror) description: An aldehyde group formula [wikipedia] for example, due to this process, we can find out what component the solution contains: Tollen's test. Silver Mirror Reaction Formula.