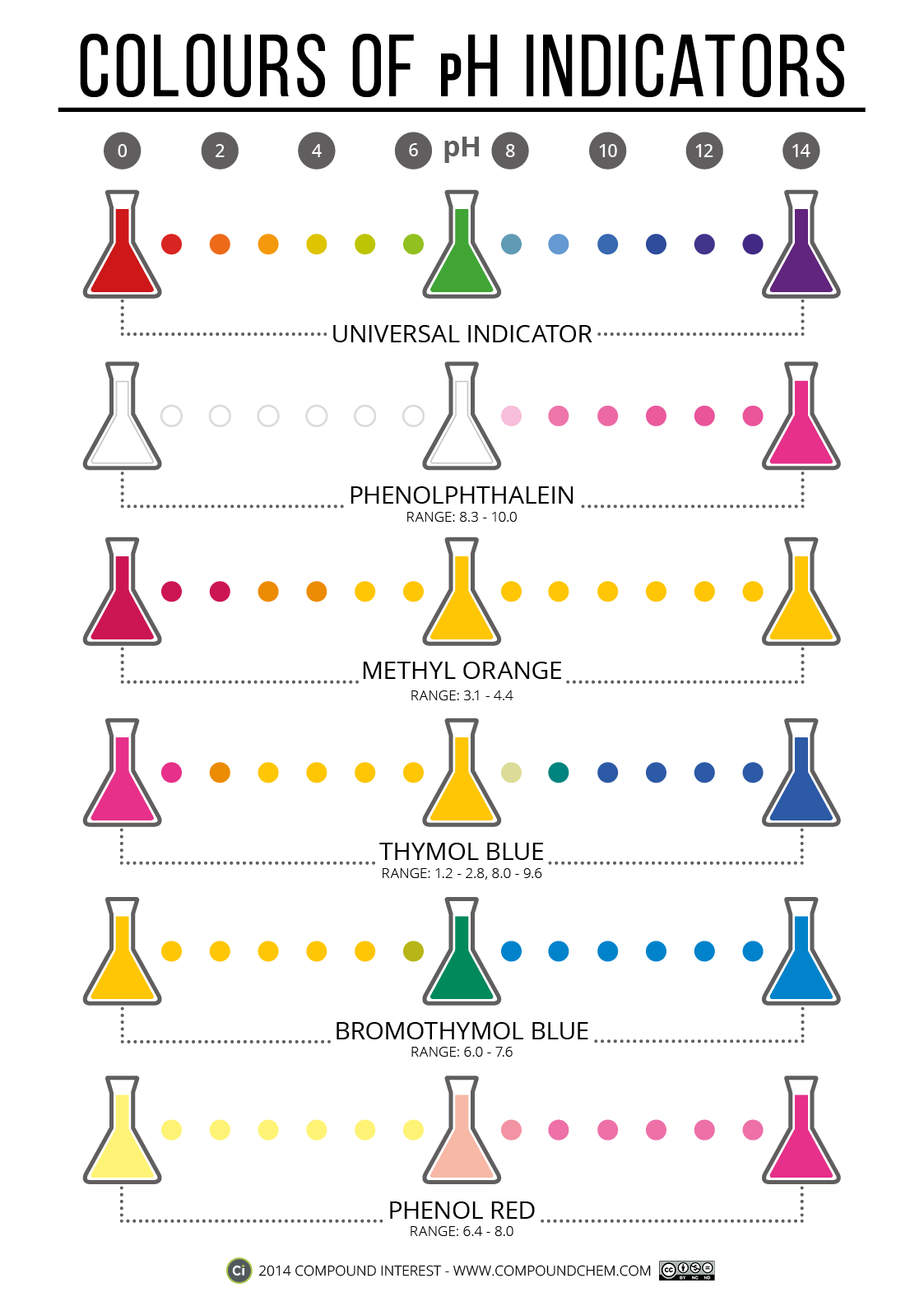
Acid Or Base Indicator Color . Certain organic substances change color in dilute solution when the hydronium ion. The next diagram shows the ph curve for adding a strong acid to a strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. They are usually weak acids or bases,. Here is a chart of. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Superimposed on it are the ph. Determine the acidic dissociation constants ka or kai of indicators. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances whose solutions change color due to changes in ph.
from sites.google.com
The undissociated form of the indicator is a different color than the iogenic form of the indicator. Superimposed on it are the ph. Determine the acidic dissociation constants ka or kai of indicators. Certain organic substances change color in dilute solution when the hydronium ion. They are usually weak acids or bases,. The next diagram shows the ph curve for adding a strong acid to a strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Here is a chart of.
AP Unit 6 Acid Base Equilibria Baumann Chemistry
Acid Or Base Indicator Color When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Superimposed on it are the ph. Here is a chart of. The next diagram shows the ph curve for adding a strong acid to a strong base. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Determine the acidic dissociation constants ka or kai of indicators. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases,. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Certain organic substances change color in dilute solution when the hydronium ion.
From www.alamy.com
Phenolphthalein is used as a single indicator in acidbase titrations Acid Or Base Indicator Color The next diagram shows the ph curve for adding a strong acid to a strong base. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The undissociated form of the indicator is a different color than the iogenic form of the indicator. In an acidic solution, the colour indicator. Acid Or Base Indicator Color.
From www.goodscience.com.au
Acids, Bases and pH Good Science Acid Or Base Indicator Color When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The next diagram shows the ph curve for adding a strong acid to a strong base. Determine the acidic dissociation constants ka or kai of indicators. Superimposed on it are the ph. Indicators are substances whose solutions change color due. Acid Or Base Indicator Color.
From dxokymive.blob.core.windows.net
Types Of Indicators In Acid Base Titration at Donna Gutierrez blog Acid Or Base Indicator Color Determine the acidic dissociation constants ka or kai of indicators. The next diagram shows the ph curve for adding a strong acid to a strong base. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. The undissociated form of the. Acid Or Base Indicator Color.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Acid Or Base Indicator Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Here is a chart of. Indicators are substances whose solutions change. Acid Or Base Indicator Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Acid Or Base Indicator Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances whose solutions change color due to changes in ph. Here is a chart of. Determine the acidic dissociation constants ka or kai of indicators. The next diagram shows. Acid Or Base Indicator Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Acid Or Base Indicator Color The undissociated form of the indicator is a different color than the iogenic form of the indicator. They are usually weak acids or bases,. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Here is a chart of. Superimposed on. Acid Or Base Indicator Color.
From pediaa.com
Difference Between Acid Base Indicator and Universal Indicator Acid Or Base Indicator Color They are usually weak acids or bases,. Superimposed on it are the ph. Indicators are substances whose solutions change color due to changes in ph. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Determine the acidic dissociation constants ka or kai of indicators. In an acidic solution, the. Acid Or Base Indicator Color.
From www.pinterest.ch
Pin on School Acid Or Base Indicator Color Certain organic substances change color in dilute solution when the hydronium ion. The undissociated form of the indicator is a different color than the iogenic form of the indicator. The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph. When titrating strong acids or bases, aim for a. Acid Or Base Indicator Color.
From foundoutaboutchemistry.blogspot.com
Found Out About Chemistry Acidbase indicator charts Acid Or Base Indicator Color Here is a chart of. Indicators are substances whose solutions change color due to changes in ph. Determine the acidic dissociation constants ka or kai of indicators. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The undissociated form of the indicator is a different color than the iogenic. Acid Or Base Indicator Color.
From knowledge.carolina.com
AcidBase Indicators Carolina Knowledge Center Acid Or Base Indicator Color The undissociated form of the indicator is a different color than the iogenic form of the indicator. The next diagram shows the ph curve for adding a strong acid to a strong base. Certain organic substances change color in dilute solution when the hydronium ion. Here is a chart of. When titrating strong acids or bases, aim for a ph. Acid Or Base Indicator Color.
From theory.labster.com
SäureBasenIndikatorTabelle Labster Theory Acid Or Base Indicator Color The undissociated form of the indicator is a different color than the iogenic form of the indicator. Determine the acidic dissociation constants ka or kai of indicators. They are usually weak acids or bases,. Certain organic substances change color in dilute solution when the hydronium ion. When titrating strong acids or bases, aim for a ph indicator that displays a. Acid Or Base Indicator Color.
From ar.inspiredpencil.com
Ph Indicator Chart Acid Or Base Indicator Color Determine the acidic dissociation constants ka or kai of indicators. Certain organic substances change color in dilute solution when the hydronium ion. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The undissociated form of the indicator is a different color than the iogenic form of the indicator. The. Acid Or Base Indicator Color.
From courses.lumenlearning.com
AcidBase Indicators Introduction to Chemistry Acid Or Base Indicator Color The next diagram shows the ph curve for adding a strong acid to a strong base. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Certain organic substances change color in dilute solution when the hydronium ion. When titrating strong. Acid Or Base Indicator Color.
From www.chemedx.org
Making Natural Acid Base Indicators Chemical Education Xchange Acid Or Base Indicator Color Superimposed on it are the ph. Indicators are substances whose solutions change color due to changes in ph. They are usually weak acids or bases,. Here is a chart of. Certain organic substances change color in dilute solution when the hydronium ion. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a. Acid Or Base Indicator Color.
From www.chegg.com
Solved (15) Determine the color of the solution based on the Acid Or Base Indicator Color Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Superimposed on it are the ph. Certain organic substances change color in dilute solution when the hydronium ion. The. Acid Or Base Indicator Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Acid Or Base Indicator Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Indicators are substances whose solutions change color due to changes in ph. Here is a chart of. They are usually weak acids or bases,. The undissociated form of the indicator is. Acid Or Base Indicator Color.
From www.teachoo.com
Acid Base Indicators All types [List with Examples] Teachoo Acid Or Base Indicator Color The next diagram shows the ph curve for adding a strong acid to a strong base. Indicators are substances whose solutions change color due to changes in ph. Determine the acidic dissociation constants ka or kai of indicators. The undissociated form of the indicator is a different color than the iogenic form of the indicator. Superimposed on it are the. Acid Or Base Indicator Color.
From www.freepik.com
Free Vector A ph scale on white background Acid Or Base Indicator Color They are usually weak acids or bases,. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Certain organic substances change color in dilute solution when the hydronium ion. Here is a chart of. The undissociated form of the indicator is a different color than the iogenic form of the. Acid Or Base Indicator Color.
From www.dreamstime.com
PH Indicator. Color Change of Blue Litmus Paper To Red for Acids, Red Acid Or Base Indicator Color When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Determine the acidic dissociation constants ka or kai of indicators. Superimposed on it are the ph. Here is a chart of. The next diagram shows the ph curve for adding a strong acid to a strong base. Indicators are substances. Acid Or Base Indicator Color.
From www.chemistry4students.com
Chemistry 4 Students Common AcidBase Indicators Acid Or Base Indicator Color Certain organic substances change color in dilute solution when the hydronium ion. They are usually weak acids or bases,. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Superimposed on it are the ph. Indicators are substances whose solutions change. Acid Or Base Indicator Color.
From www.howtopronounce.com
Take the free online AcidBase Indicators Color Change! Chemistry Acid Or Base Indicator Color Here is a chart of. The next diagram shows the ph curve for adding a strong acid to a strong base. Superimposed on it are the ph. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. The undissociated form of the indicator is a different color than the iogenic. Acid Or Base Indicator Color.
From fineartamerica.com
Phenolphthalein Acidbase Indicator Photograph by Science Photo Library Acid Or Base Indicator Color They are usually weak acids or bases,. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Certain organic substances change color in dilute solution when the hydronium ion. Determine the acidic dissociation constants ka or kai of indicators. The next. Acid Or Base Indicator Color.
From www.chegg.com
Solved (15) Determine the color of the solution based on the Acid Or Base Indicator Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Here is a chart of. The next diagram shows the ph curve for adding a strong acid to a strong base. Indicators are substances whose solutions change color due to changes. Acid Or Base Indicator Color.
From davidswart.org
Acid and Base Lab with Report The Art of Science Acid Or Base Indicator Color Determine the acidic dissociation constants ka or kai of indicators. Here is a chart of. Superimposed on it are the ph. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Certain organic substances change color in dilute solution when the hydronium ion. They are usually weak acids or bases,.. Acid Or Base Indicator Color.
From www.dreamstime.com
Ph Acid and Base Indicator Color Chart Stock Image Image of paper Acid Or Base Indicator Color They are usually weak acids or bases,. Determine the acidic dissociation constants ka or kai of indicators. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances whose solutions change color due to changes in ph. The next diagram shows the ph curve for adding a strong. Acid Or Base Indicator Color.
From iteachly.com
Acid and Base Indicators Lab Activity ⋆ Acid Or Base Indicator Color They are usually weak acids or bases,. The undissociated form of the indicator is a different color than the iogenic form of the indicator. The next diagram shows the ph curve for adding a strong acid to a strong base. Determine the acidic dissociation constants ka or kai of indicators. Here is a chart of. Superimposed on it are the. Acid Or Base Indicator Color.
From ar.inspiredpencil.com
Acids And Bases List Strength Acid Or Base Indicator Color Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Superimposed on it are the ph. They are usually weak acids or bases,. The undissociated form of the indicator. Acid Or Base Indicator Color.
From www.slideserve.com
PPT Properties of Acids PowerPoint Presentation, free download ID Acid Or Base Indicator Color They are usually weak acids or bases,. Indicators are substances whose solutions change color due to changes in ph. Here is a chart of. Superimposed on it are the ph. Certain organic substances change color in dilute solution when the hydronium ion. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as. Acid Or Base Indicator Color.
From sciencenotes.org
pH Indicator Chart Colors and Ranges Acid Or Base Indicator Color When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Indicators are substances whose solutions change color due to changes in ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to. Acid Or Base Indicator Color.
From nigerianscholars.com
AcidBase Indicators AcidBase Equilibria Acid Or Base Indicator Color Determine the acidic dissociation constants ka or kai of indicators. Superimposed on it are the ph. The next diagram shows the ph curve for adding a strong acid to a strong base. The undissociated form of the indicator is a different color than the iogenic form of the indicator. In an acidic solution, the colour indicator will exist in its. Acid Or Base Indicator Color.
From www.myxxgirl.com
Science Experiment Acid Base Indicator How To Identify Acids Bases My Acid Or Base Indicator Color In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. Certain organic substances change color in dilute solution when the hydronium ion. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral. Acid Or Base Indicator Color.
From www.youtube.com
ACID BASE INDICATORS YouTube Acid Or Base Indicator Color Superimposed on it are the ph. Determine the acidic dissociation constants ka or kai of indicators. The next diagram shows the ph curve for adding a strong acid to a strong base. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to. Acid Or Base Indicator Color.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive Acid Or Base Indicator Color Superimposed on it are the ph. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it is converted to its. They are usually weak acids or bases,. Indicators are substances whose solutions change color due to changes in ph. Determine the acidic dissociation constants ka. Acid Or Base Indicator Color.
From chem.libretexts.org
17.3 AcidBase Indicators Chemistry LibreTexts Acid Or Base Indicator Color Determine the acidic dissociation constants ka or kai of indicators. The next diagram shows the ph curve for adding a strong acid to a strong base. Here is a chart of. Superimposed on it are the ph. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Certain organic substances. Acid Or Base Indicator Color.
From sites.google.com
AP Unit 6 Acid Base Equilibria Baumann Chemistry Acid Or Base Indicator Color Here is a chart of. When titrating strong acids or bases, aim for a ph indicator that displays a color change near a neutral ph. Determine the acidic dissociation constants ka or kai of indicators. In an acidic solution, the colour indicator will exist in its conjugate acid form (one colour), but as base is added and ph increases, it. Acid Or Base Indicator Color.