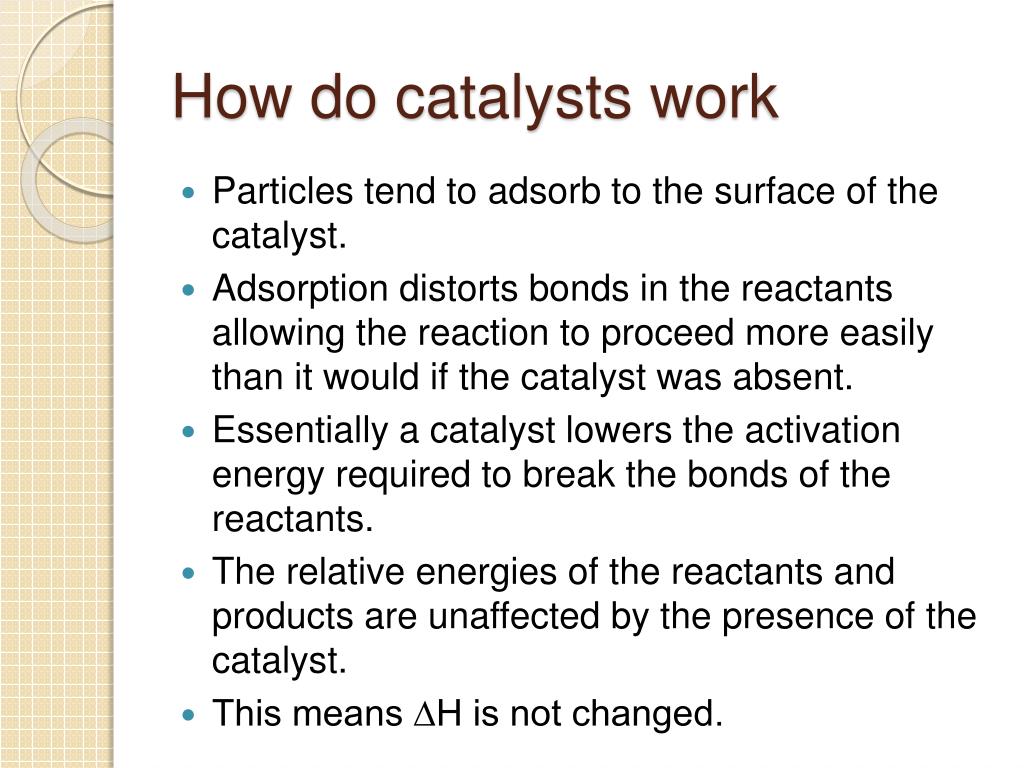
Catalysts At Work Quizlet . Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. A place where the reaction occurs. A substance that increases the rate of a reaction without being used up during the reaction is called a. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A place on the product where the enzyme fits. A molecule that acts as a catalyst. In heterogeneous catalysis, catalysts provide a surface to. List examples of catalysis in natural. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Ethene gas reacts with hydrogen gas by using a nickel catalyst. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Consider the energy diagram below. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Which statement best describes the.
from www.slideserve.com
Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A molecule that acts as a catalyst. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. List examples of catalysis in natural. A substance that increases the rate of a reaction without being used up during the reaction is called a. A place on the product where the enzyme fits. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A place where the reaction occurs. Consider the energy diagram below.
PPT Fast and Slow Chemistry PowerPoint Presentation, free download
Catalysts At Work Quizlet Consider the energy diagram below. A place where the reaction occurs. In heterogeneous catalysis, catalysts provide a surface to. Which statement best describes the. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. A substance that increases the rate of a reaction without being used up during the reaction is called a. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A place on the product where the enzyme fits. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. List examples of catalysis in natural. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A molecule that acts as a catalyst. Consider the energy diagram below. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.
From quizlet.com
catalysts Diagram Quizlet Catalysts At Work Quizlet In heterogeneous catalysis, catalysts provide a surface to. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. A substance that increases the rate of a reaction without being used up during the reaction is called a. Which statement best describes the. A catalyst is a chemical substance that. Catalysts At Work Quizlet.
From www.slideserve.com
PPT Chapter 15 Fast and Slow Chemistry PowerPoint Presentation Catalysts At Work Quizlet Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A substance that increases the rate of a reaction without being used up during the reaction is called a. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Study with quizlet and memorize flashcards containing. Catalysts At Work Quizlet.
From www.youtube.com
Quizlet 101 An introduction to Quizlet for teachers and students YouTube Catalysts At Work Quizlet Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. A catalyst is a chemical. Catalysts At Work Quizlet.
From socratic.org
Why does a catalyst cause a reaction to speed up? Socratic Catalysts At Work Quizlet A substance that increases the rate of a reaction without being used up during the reaction is called a. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A place on the product where the enzyme fits. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,.. Catalysts At Work Quizlet.
From studylib.net
Catalysts at work Catalysts At Work Quizlet Consider the energy diagram below. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. A molecule that acts as a catalyst. Which statement best describes the. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the. Catalysts At Work Quizlet.
From quizlet.com
Humor and Graphic Art as Catalysts Towards Climate Justice SOLUTIONS Catalysts At Work Quizlet A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. List. Catalysts At Work Quizlet.
From www.slideserve.com
PPT Catalysts PowerPoint Presentation, free download ID2229930 Catalysts At Work Quizlet List examples of catalysis in natural. A molecule that acts as a catalyst. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A place on the product where the enzyme fits. In heterogeneous catalysis, catalysts provide a surface to. Which statement best describes the. Consider the energy diagram below. A catalyst is a chemical. Catalysts At Work Quizlet.
From www.globalspec.com
Catalysts and Initiators Information Engineering360 Catalysts At Work Quizlet In heterogeneous catalysis, catalysts provide a surface to. List examples of catalysis in natural. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Which statement best describes the. A catalyst is a chemical substance that. Catalysts At Work Quizlet.
From courses.lumenlearning.com
Catalysis Chemistry Catalysts At Work Quizlet List examples of catalysis in natural. Consider the energy diagram below. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. Ethene gas reacts with hydrogen gas by using a nickel catalyst. In heterogeneous catalysis, catalysts provide a surface to. A place where the reaction occurs. Catalysts allow a. Catalysts At Work Quizlet.
From www.savemyexams.com
Catalysts & Reaction Rate College Board AP Chemistry Revision Notes 2022 Catalysts At Work Quizlet Ethene gas reacts with hydrogen gas by using a nickel catalyst. A place on the product where the enzyme fits. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A substance that increases the rate of a reaction without being used up during the reaction is called a. The greater the frequency of successful. Catalysts At Work Quizlet.
From studylib.net
How do Catalysts Work? Lab Catalysts At Work Quizlet Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A substance that increases the rate of a reaction without being used up during the reaction is called a. A molecule that acts as a catalyst. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina. Catalysts At Work Quizlet.
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii Catalysts At Work Quizlet Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Consider the energy diagram below. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for. Catalysts At Work Quizlet.
From exyqggorv.blob.core.windows.net
Enzymes Are Proteins That Act As Biological Catalysts Quizlet at Alice Catalysts At Work Quizlet A place on the product where the enzyme fits. Consider the energy diagram below. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Study with quizlet and memorize flashcards containing terms like what is a catalyst?,. Catalysts At Work Quizlet.
From slideplayer.com
ENZYMES VOCABULARY PLUS WORKSHEET ppt download Catalysts At Work Quizlet In heterogeneous catalysis, catalysts provide a surface to. A molecule that acts as a catalyst. Ethene gas reacts with hydrogen gas by using a nickel catalyst. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Consider the energy diagram below. A substance that increases the rate of a reaction without. Catalysts At Work Quizlet.
From www.slideserve.com
PPT Chapter 15 Fast and Slow Chemistry PowerPoint Presentation Catalysts At Work Quizlet A place where the reaction occurs. List examples of catalysis in natural. In heterogeneous catalysis, catalysts provide a surface to. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed.. Catalysts At Work Quizlet.
From www.scribd.com
How Do Catalysts Work PDF Catalysis Chemical Reactions Catalysts At Work Quizlet In heterogeneous catalysis, catalysts provide a surface to. A place where the reaction occurs. Consider the energy diagram below. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The. Catalysts At Work Quizlet.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download Catalysts At Work Quizlet The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. List examples of catalysis in natural. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A substance that increases the rate of. Catalysts At Work Quizlet.
From www.youtube.com
What is a catalyst and how does catalysis work? YouTube Catalysts At Work Quizlet The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Which statement best describes the. List examples of catalysis in natural. A substance that increases the rate of a reaction without being used up during the reaction is called a. A molecule that acts as a catalyst. In heterogeneous catalysis, catalysts provide a surface to.. Catalysts At Work Quizlet.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of Catalysts At Work Quizlet A molecule that acts as a catalyst. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A substance that increases the rate of a reaction without being used up during the reaction is called a. In heterogeneous catalysis, catalysts provide a surface to. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate.. Catalysts At Work Quizlet.
From www.pinterest.com
Catalyst Easy Science Energy activities, Chemical reactions Catalysts At Work Quizlet A molecule that acts as a catalyst. A substance that increases the rate of a reaction without being used up during the reaction is called a. Ethene gas reacts with hydrogen gas by using a nickel catalyst. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. The greater the frequency of successful collisions between. Catalysts At Work Quizlet.
From www.mdpi.com
Catalysts Free FullText Recent Advances in Electrochemical Catalysts At Work Quizlet Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A molecule that acts as a catalyst. A substance that increases the rate of a reaction without being used up during the reaction is called a. Explain the function of. Catalysts At Work Quizlet.
From giokorkmx.blob.core.windows.net
Enzymes That Function As Catalysts at Jeffrey Timms blog Catalysts At Work Quizlet A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. Consider the energy diagram below. A place on the product where the enzyme fits.. Catalysts At Work Quizlet.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download Catalysts At Work Quizlet The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A place on the product where the enzyme fits. A molecule that acts as a catalyst. Which statement best describes the. Ethene gas reacts with hydrogen gas by using a nickel catalyst. A place where the reaction occurs. Consider the energy diagram below. Catalysts allow. Catalysts At Work Quizlet.
From www.youtube.com
How does a CATALYST work ? YouTube Catalysts At Work Quizlet Ethene gas reacts with hydrogen gas by using a nickel catalyst. A molecule that acts as a catalyst. In heterogeneous catalysis, catalysts provide a surface to. A substance that increases the rate of a reaction without being used up during the reaction is called a. A catalyst is a chemical substance that affects the rate of a chemical reaction by. Catalysts At Work Quizlet.
From www.linstitute.net
IB DP Chemistry HL复习笔记6.1.8 Energy Profiles & Catalysis翰林国际教育 Catalysts At Work Quizlet A substance that increases the rate of a reaction without being used up during the reaction is called a. Consider the energy diagram below. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Which statement best describes the. Study with quizlet and memorize flashcards containing terms like what is a. Catalysts At Work Quizlet.
From dokumen.tips
(PDF) Catalysts and How They Work DOKUMEN.TIPS Catalysts At Work Quizlet The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A place where the reaction occurs. Consider the energy diagram below. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Which statement best describes the. Explain the function of a catalyst in terms of reaction. Catalysts At Work Quizlet.
From www.tffn.net
How Does Catalyst Work? A Comprehensive Guide to Understanding Catalysts At Work Quizlet A molecule that acts as a catalyst. A substance that increases the rate of a reaction without being used up during the reaction is called a. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical reaction?,. Catalysts allow a reaction to proceed via a pathway that has a lower. Catalysts At Work Quizlet.
From www.cheric.org
Chemical Reaction (Reaction rate) Catalysts At Work Quizlet A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A molecule that acts as a catalyst. A place where the reaction occurs. Which statement best describes the. Ethene gas. Catalysts At Work Quizlet.
From blog.syrris.com
Solid phase catalysis in continuous flow Syrris chemistry blog Catalysts At Work Quizlet A substance that increases the rate of a reaction without being used up during the reaction is called a. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. List. Catalysts At Work Quizlet.
From bio1151.nicerweb.com
catalytic.html 08_17CatalyticCycle.jpg Catalysts At Work Quizlet Which statement best describes the. A molecule that acts as a catalyst. A place on the product where the enzyme fits. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical. Catalysts At Work Quizlet.
From eng.nju.edu.cn
Binuclear Cu complex catalysis enabling Li Catalysts At Work Quizlet List examples of catalysis in natural. A place where the reaction occurs. A substance that increases the rate of a reaction without being used up during the reaction is called a. Consider the energy diagram below. Which statement best describes the. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction.. Catalysts At Work Quizlet.
From alchetron.com
Heterogeneous catalysis Alchetron, the free social encyclopedia Catalysts At Work Quizlet Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Consider the energy diagram below. The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. A place where the reaction occurs. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams.. Catalysts At Work Quizlet.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis Catalysts At Work Quizlet The greater the frequency of successful collisions between reactant particles, the greater the reaction rate. Ethene gas reacts with hydrogen gas by using a nickel catalyst. In heterogeneous catalysis, catalysts provide a surface to. A catalyst is a chemical substance that affects the rate of a chemical reaction by altering the activation energy required for the reaction to proceed. A. Catalysts At Work Quizlet.
From www.gamepur.com
How do Quizlet codes work? How to use Quizlet.live codes Gamepur Catalysts At Work Quizlet Catalysts allow a reaction to proceed via a pathway that has a lower activation energy than the uncatalyzed reaction. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A place where the reaction occurs. Study with quizlet and memorize flashcards containing terms like what is a catalyst?, does a catalyst get altered ina chemical. Catalysts At Work Quizlet.
From quizlet.com
Vince worked 18 hours last week. Part of the time he worked Quizlet Catalysts At Work Quizlet In heterogeneous catalysis, catalysts provide a surface to. Ethene gas reacts with hydrogen gas by using a nickel catalyst. Which statement best describes the. Explain the function of a catalyst in terms of reaction mechanisms and potential energy diagrams. A place where the reaction occurs. Catalysts allow a reaction to proceed via a pathway that has a lower activation energy. Catalysts At Work Quizlet.