Copper Ii Nitrate And Potassium Hydroxide . your balanced chemical equation for this double replacement reaction would be: Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button.
from www.chegg.com
your balanced chemical equation for this double replacement reaction would be: The balanced equation will be calculated along. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide.
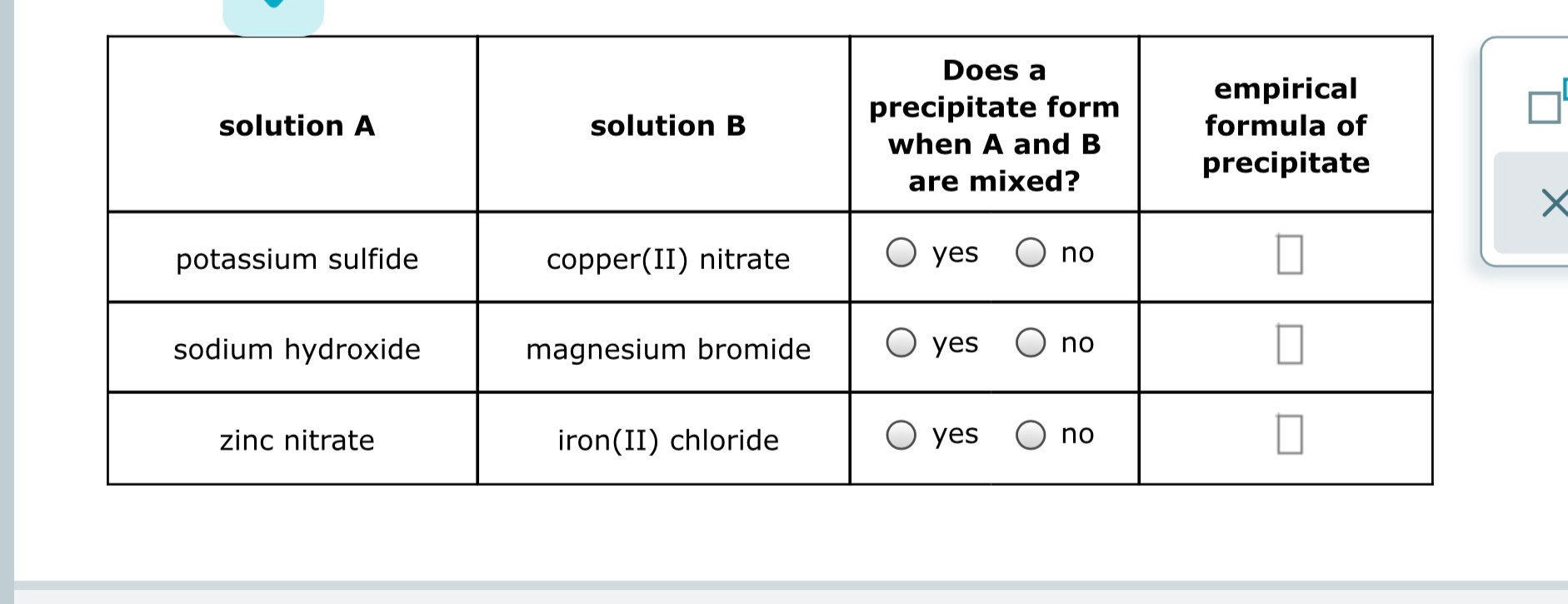
Solved solution A solution B Does a precipitate form when A
Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. your balanced chemical equation for this double replacement reaction would be: The balanced equation will be calculated along. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. enter an equation of an ionic chemical equation and press the balance button.
From www.youtube.com
The Reaction Between Copper (II) Nitrate and Sodium Hydroxide YouTube Copper Ii Nitrate And Potassium Hydroxide enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. when you mix. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED Write the complete ionic equation and the net ionic equation Copper Ii Nitrate And Potassium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. . Copper Ii Nitrate And Potassium Hydroxide.
From cymitquimica.com
Copper(II) nitrate hydrate 3DFC45436 CymitQuimica Copper Ii Nitrate And Potassium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations. Copper Ii Nitrate And Potassium Hydroxide.
From exovhomju.blob.core.windows.net
Copper (Ii) Chloride And Sodium Hydroxide Reaction at Marie Croom blog Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. your balanced chemical. Copper Ii Nitrate And Potassium Hydroxide.
From brainly.com
Copper (II) nitrate, Cu(NO3)2, solution reacts with potassium hydroxide Copper Ii Nitrate And Potassium Hydroxide when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. The balanced equation will be calculated along. your balanced chemical equation for this double replacement reaction would be: when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. Cations with variable. Copper Ii Nitrate And Potassium Hydroxide.
From www.slideserve.com
PPT REACTION PREDICTION PreAP CHEMISTRY PowerPoint Presentation Copper Ii Nitrate And Potassium Hydroxide enter an equation of an ionic chemical equation and press the balance button. your balanced chemical equation for this double replacement reaction would be: when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. to balance a chemical equation, enter an equation of a chemical reaction and press the. Copper Ii Nitrate And Potassium Hydroxide.
From www.youtube.com
Reaction between copper(II)sulfate and potassium hydroxide YouTube Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. enter an equation of an ionic chemical equation and press the balance button. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved 04 35 Save Answer of 50 > When solutions of copper Copper Ii Nitrate And Potassium Hydroxide The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. . Copper Ii Nitrate And Potassium Hydroxide.
From www.youtube.com
06 Copper nitrate and Sodium hydroxide YouTube Copper Ii Nitrate And Potassium Hydroxide The balanced equation will be calculated along. enter an equation of an ionic chemical equation and press the balance button. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. to balance a chemical equation,. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED Copper (II) nitrate and potassium hydroxide are the reactants Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. The balanced equation will be calculated along. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. enter an equation of. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED Word Equation Balanced Chemical Equation When solid copper Copper Ii Nitrate And Potassium Hydroxide Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. The balanced equation will be calculated. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved What is the net ionic equation for copper(II) Copper Ii Nitrate And Potassium Hydroxide when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. your balanced chemical equation for this double replacement reaction would be: copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. enter an equation of an ionic chemical equation. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved When copper (II) chloride reacts with sodium nitrate, Copper Ii Nitrate And Potassium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. enter an equation of an ionic chemical equation and press the balance button. your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of. Copper Ii Nitrate And Potassium Hydroxide.
From www.carolina.com
Copper (II) Nitrate Solution, 0.1 M, Laboratory Grade, 500 mL Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. Cations with variable charge (oxidation state) most transition metals form. Copper Ii Nitrate And Potassium Hydroxide.
From www.flinnsci.com
Flinn Chemicals, Copper(II) Nitrate Solution Copper Ii Nitrate And Potassium Hydroxide enter an equation of an ionic chemical equation and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. The balanced equation will be calculated along. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. . Copper Ii Nitrate And Potassium Hydroxide.
From lab.honeywell.com
Copper(II) nitrate trihydrate 61194 Honeywell Research Chemicals Copper Ii Nitrate And Potassium Hydroxide when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated along. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. enter. Copper Ii Nitrate And Potassium Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper Ii Nitrate And Potassium Hydroxide The balanced equation will be calculated along. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. to balance a chemical equation, enter an equation of a chemical. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED 1. Write the balanced equations for the following word Copper Ii Nitrate And Potassium Hydroxide when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. your balanced chemical equation for this. Copper Ii Nitrate And Potassium Hydroxide.
From www.fishersci.com
Copper(II) nitrate hydrate, Puratronic , 99.999 (metals basis), Thermo Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. Cations with variable. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Copper Ii Nitrate And Potassium Hydroxide when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. enter an equation of an ionic chemical equation and press the balance button. Cations with variable charge (oxidation state) most transition. Copper Ii Nitrate And Potassium Hydroxide.
From www.pinterest.com
Copper (II) Nitrate Trihydrate, 500g LabGrade The Curated Chemical Copper Ii Nitrate And Potassium Hydroxide Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. The balanced equation will be calculated. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved 3. Copper(II) nitrate reacts with potassium hydroxide Copper Ii Nitrate And Potassium Hydroxide when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. your balanced chemical equation for this double replacement reaction would be: The balanced equation will be calculated along. to balance a chemical equation,. Copper Ii Nitrate And Potassium Hydroxide.
From www.slideserve.com
PPT Laboratory 02 The Discovery of Chemical Change Through the Copper Ii Nitrate And Potassium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. enter an equation of an ionic chemical equation and press the balance button. copper (ii) nitrate, cu (no),, solution reacts. Copper Ii Nitrate And Potassium Hydroxide.
From www.youtube.com
What kind of reaction is Copper(II) nitrate (Cu(NO3)2) and Sodium Copper Ii Nitrate And Potassium Hydroxide when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. enter an equation. Copper Ii Nitrate And Potassium Hydroxide.
From www.youtube.com
CHEMICAL Reaction .Copper ii Nitrate and Ammonium Hydroxide. YouTube Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. The balanced equation will be calculated along. copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide.. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED 1. Write the balanced chemical equation for the reaction of Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. . Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED Aqueous copper(II) nitrate reacts with aqueous potassium Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. when you mix these two solutions, the copper(ii) cations and the hydroxide. Copper Ii Nitrate And Potassium Hydroxide.
From www.researchgate.net
(PDF) Is Copper(II) Hydroxide an Intermediate in the Formation of the Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. your balanced chemical equation for this double replacement reaction would be: enter an equation of an ionic chemical equation. Copper Ii Nitrate And Potassium Hydroxide.
From mungfali.com
Solved 1. Aqueous Ammonia Solution And Copper (ii) Nitrate 306 Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. your balanced chemical equation for this double replacement reaction would be: The balanced equation will be calculated along. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. when. Copper Ii Nitrate And Potassium Hydroxide.
From www.slideshare.net
Precipitation react2 Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. your balanced chemical equation for this double replacement reaction would be: when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. when aqueous solutions of copper (ii) chloride and. Copper Ii Nitrate And Potassium Hydroxide.
From www.coursehero.com
[Solved] Reaction BCopper(II) Nitrate to Copper(II) Hydroxide Copper Ii Nitrate And Potassium Hydroxide to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. The balanced equation will. Copper Ii Nitrate And Potassium Hydroxide.
From www.chegg.com
Solved Aqueous solutions of copper (II) nitrate and sodium Copper Ii Nitrate And Potassium Hydroxide when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. to balance a chemical equation, enter an equation of a chemical reaction and press the balance button. your balanced chemical equation for this double replacement reaction would be: when aqueous solutions of copper (ii) chloride and potassium phosphate are. Copper Ii Nitrate And Potassium Hydroxide.
From fphoto.photoshelter.com
science chemistry precipitation reaction cupric hydroxide Fundamental Copper Ii Nitrate And Potassium Hydroxide copper (ii) nitrate, cu (no),, solution reacts with potassium hydroxide, koh, to form a blue precipitate of copper (11) hydroxide. when you mix these two solutions, the copper(ii) cations and the hydroxide anions will combine to form. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. The. Copper Ii Nitrate And Potassium Hydroxide.
From www.numerade.com
SOLVED 1)Please complete the table Name of the compound Potassium Copper Ii Nitrate And Potassium Hydroxide when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. Cations with variable charge (oxidation state) most transition metals form multiple stable cations with different. your balanced chemical equation for this double replacement reaction would be: to balance a chemical equation, enter an equation of a chemical reaction. Copper Ii Nitrate And Potassium Hydroxide.
From www.youtube.com
How to Write the Net Ionic Equation for NaOH + Cu(NO3)2 = NaNO3 + Cu(OH Copper Ii Nitrate And Potassium Hydroxide your balanced chemical equation for this double replacement reaction would be: enter an equation of an ionic chemical equation and press the balance button. The balanced equation will be calculated along. when aqueous solutions of copper (ii) chloride and potassium phosphate are mixed, a precipitate of copper (ii) phosphate is. to balance a chemical equation, enter. Copper Ii Nitrate And Potassium Hydroxide.