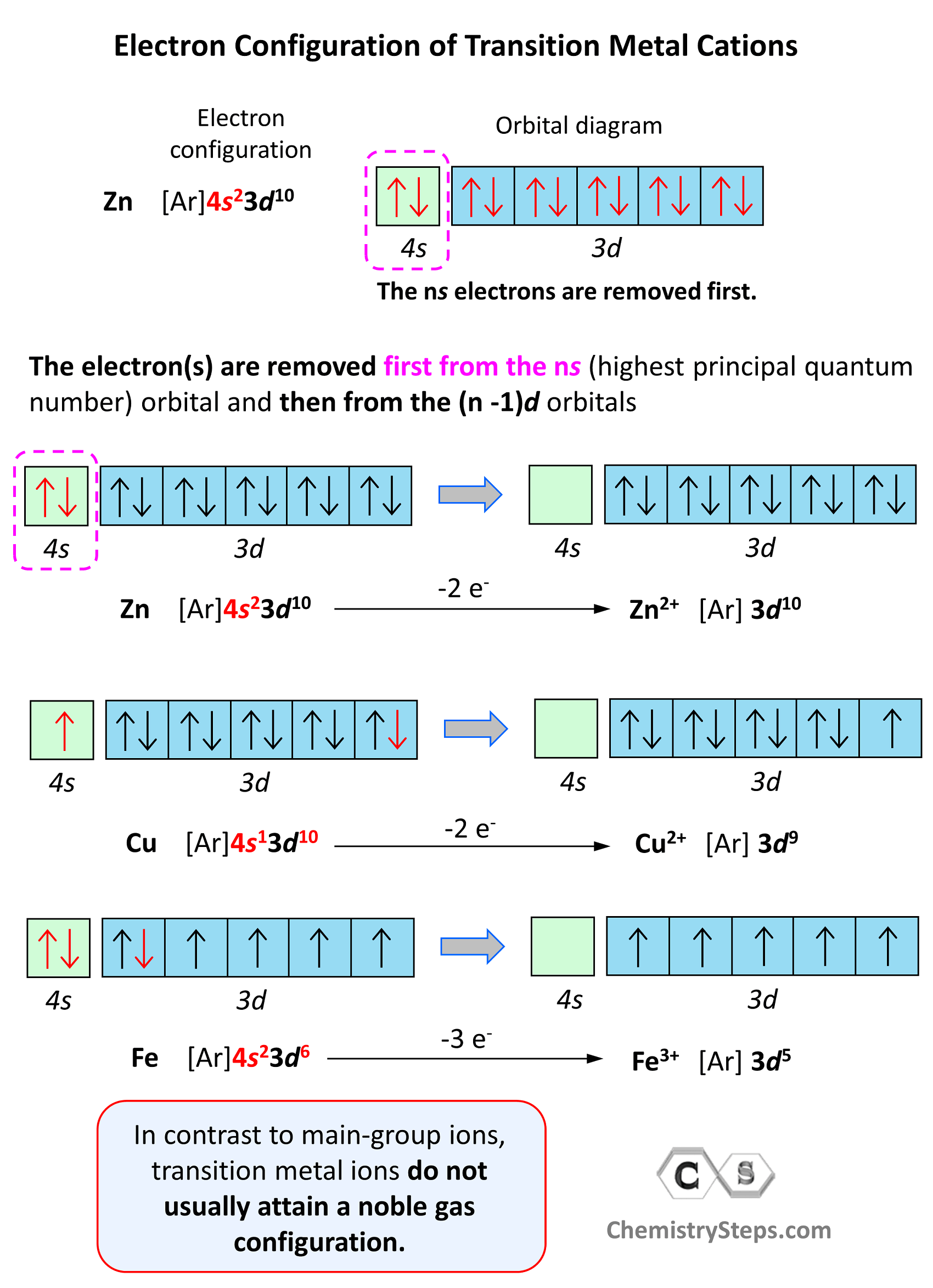
Iron Notation Electron Configuration . The electron configuration can be represented using the electrons in boxes notation. To write the electron configuration for iron, use the notation: Its valence orbitals are the #4s# and #3d# 's. Electron configuration chart of all elements is mentioned in the table below. We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The shorthand electron configuration (or noble gas configuration) as well as. The initial two electrons in the electron configuration for iron will be in the 1s orbital. How many orbitals are required for the electronic configuration of iron? The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Writing the electron configuration, you really only. Each box represents an atomic orbital. How do you write fe.
from general.chemistrysteps.com
Writing the electron configuration, you really only. Its valence orbitals are the #4s# and #3d# 's. We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. How many orbitals are required for the electronic configuration of iron? The electron configuration can be represented using the electrons in boxes notation. The shorthand electron configuration (or noble gas configuration) as well as. The initial two electrons in the electron configuration for iron will be in the 1s orbital. To write the electron configuration for iron, use the notation: The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2.
Electron Configurations of Ions Chemistry Steps
Iron Notation Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. Writing the electron configuration, you really only. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. How do you write fe. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The electron configuration can be represented using the electrons in boxes notation. To write the electron configuration for iron, use the notation: Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Its valence orbitals are the #4s# and #3d# 's. Each box represents an atomic orbital. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. How many orbitals are required for the electronic configuration of iron? We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. The shorthand electron configuration (or noble gas configuration) as well as.
From www.pinterest.com
Electron Configuration Chart for the Elements Chart, Chemistry and Iron Notation Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The electron configuration can be represented using the electrons in boxes notation. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Writing the electron configuration, you really only. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The shorthand electron configuration (or. Iron Notation Electron Configuration.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. The initial two electrons in the electron configuration for iron will be in the 1s orbital. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Each box represents. Iron Notation Electron Configuration.
From ar.inspiredpencil.com
Electron Configuration Of Iron Iron Notation Electron Configuration Its valence orbitals are the #4s# and #3d# 's. The shorthand electron configuration (or noble gas configuration) as well as. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The electron configuration can be represented using the electrons in boxes notation. How many orbitals are required for the electronic configuration of iron? The. Iron Notation Electron Configuration.
From valenceelectrons.com
Electron Configuration for Iron (Fe and Fe2+, Fe3+ ions) Iron Notation Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Electron configuration chart of all elements is mentioned in the table below. To write the electron configuration for iron, use the notation: Writing the electron configuration, you really only. How many orbitals are required for the electronic configuration of iron? We will put all 26 electrons in. Iron Notation Electron Configuration.
From stock.adobe.com
Iron atomic structure has atomic number, atomic mass, electron Iron Notation Electron Configuration We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. How do you write fe. The initial two electrons in the electron configuration for iron will be in the 1s orbital. The complete electron configuration for iron. Iron Notation Electron Configuration.
From chem.libretexts.org
Electronic Configurations Intro Chemistry LibreTexts Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. How many orbitals are required for the electronic configuration of iron? Electron configuration chart of all elements is mentioned in the table below. Its core orbitals. Iron Notation Electron Configuration.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Notation Electron Configuration The electron configuration can be represented using the electrons in boxes notation. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. The shorthand electron configuration (or noble gas configuration) as well as. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Electron configuration chart of. Iron Notation Electron Configuration.
From www.siyavula.com
4.6 Electronic configuration The atom Siyavula Iron Notation Electron Configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Its valence orbitals are the #4s# and #3d# 's. Writing the electron configuration, you really only. To write the electron configuration for iron, use the notation: The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Each. Iron Notation Electron Configuration.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Notation Electron Configuration Each box represents an atomic orbital. Electron configuration chart of all elements is mentioned in the table below. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. We will put all 26 electrons in orbitals. Iron Notation Electron Configuration.
From commons.wikimedia.org
Original file (SVG file, nominally 334 × 254 pixels, file size 42 KB) Iron Notation Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. Its valence orbitals are the #4s# and #3d# 's. The electron configuration can be represented using the electrons in boxes notation. The initial two electrons in the electron configuration for iron will be in the 1s orbital. How do you write fe. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶.. Iron Notation Electron Configuration.
From www.youtube.com
How to Write the Atomic Orbital Diagram for Iron (Fe) YouTube Iron Notation Electron Configuration Writing the electron configuration, you really only. We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2.. Iron Notation Electron Configuration.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Notation Electron Configuration The shorthand electron configuration (or noble gas configuration) as well as. To write the electron configuration for iron, use the notation: Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. We will put all 26. Iron Notation Electron Configuration.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Iron Notation Electron Configuration To write the electron configuration for iron, use the notation: We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. Its valence orbitals are the #4s# and #3d# 's. Electron configuration chart of all elements is mentioned in the table below. Each box represents an atomic orbital. The initial two. Iron Notation Electron Configuration.
From learnwithdrscott.com
Electron Configuration Worksheet Easy Hard Science Iron Notation Electron Configuration How do you write fe. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. The shorthand electron configuration (or noble gas configuration) as well as. Each box represents an atomic orbital. The electron configuration can be represented using the electrons in boxes notation.. Iron Notation Electron Configuration.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Notation Electron Configuration The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. The electron configuration can be represented using the electrons in boxes notation. Its valence orbitals are the #4s# and #3d# 's. How many orbitals are required for the electronic configuration of iron? Its core. Iron Notation Electron Configuration.
From www.youtube.com
Electron Configuration of Transition Metals Iron Fe Cobalt Co and Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. Each box represents an atomic orbital. Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. The electron configuration can be represented using. Iron Notation Electron Configuration.
From chem.libretexts.org
Chapter 2.7 Electronic Structure of the Transition Metals Chemistry Iron Notation Electron Configuration How many orbitals are required for the electronic configuration of iron? To write the electron configuration for iron, use the notation: How do you write fe. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. The electron configuration can be represented using the. Iron Notation Electron Configuration.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Iron Notation Electron Configuration The initial two electrons in the electron configuration for iron will be in the 1s orbital. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but. Iron Notation Electron Configuration.
From c-laing0811.blogspot.com
Electron Configuration Using Spdf Notation Solved References Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. Its valence orbitals are the #4s# and #3d# 's. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6. Iron Notation Electron Configuration.
From pnghut.com
Electron Configuration Atomic Orbital Shell Energy Level Iron Iron Notation Electron Configuration The initial two electrons in the electron configuration for iron will be in the 1s orbital. We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar]. Iron Notation Electron Configuration.
From alquilercastilloshinchables.info
8 Photos Iron Periodic Table And Description Alqu Blog Iron Notation Electron Configuration Electron configuration chart of all elements is mentioned in the table below. The shorthand electron configuration (or noble gas configuration) as well as. To write the electron configuration for iron, use the notation: Its valence orbitals are the #4s# and #3d# 's. How many orbitals are required for the electronic configuration of iron? Commonly, the electron configuration is used to. Iron Notation Electron Configuration.
From www.youtube.com
Electron Configuration for Fe, Fe2+, and Fe3+ (Iron and Iron Ions Iron Notation Electron Configuration Electron configuration chart of all elements is mentioned in the table below. How do you write fe. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Its valence orbitals are the #4s# and #3d# 's. Writing the electron configuration, you really only. Its. Iron Notation Electron Configuration.
From www.nuclear-power.com
Iron Atomic Number Atomic Mass Density of Iron Iron Notation Electron Configuration Each box represents an atomic orbital. The shorthand electron configuration (or noble gas configuration) as well as. Electron configuration chart of all elements is mentioned in the table below. How many orbitals are required for the electronic configuration of iron? The electron configuration can be represented using the electrons in boxes notation. The initial two electrons in the electron configuration. Iron Notation Electron Configuration.
From www.numerade.com
SOLVED Use the Relcrtncc acr Mmenn Write the complete electron Iron Notation Electron Configuration To write the electron configuration for iron, use the notation: Each box represents an atomic orbital. The complete electron configuration for iron should be written as 1s2 2s2 2p6 3s2 3p6 4s2 3d6 and the abbreviated electron configuration is [ar] 3d6 4s2. Writing the electron configuration, you really only. The initial two electrons in the electron configuration for iron will. Iron Notation Electron Configuration.
From sciencenotes.org
List of Electron Configurations of Elements Iron Notation Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. To write the electron configuration for iron, use the notation: We will put. Iron Notation Electron Configuration.
From www.bartleby.com
Answered Write the complete electron… bartleby Iron Notation Electron Configuration Its valence orbitals are the #4s# and #3d# 's. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we. Iron Notation Electron Configuration.
From wisc.pb.unizin.org
Electron Configurations, Orbital Box Notation (M7Q7) UWMadison Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. How do you write fe. The electron configuration can be represented using the electrons in boxes notation. To write the electron configuration for iron, use the. Iron Notation Electron Configuration.
From homedeso.vercel.app
Group 1 Periodic Table Electron Configuration Iron Notation Electron Configuration We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. How do you write fe. Its valence orbitals are the #4s# and #3d# 's. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Writing the electron configuration, you really only. To write the electron configuration for iron, use the notation: The electron. Iron Notation Electron Configuration.
From www.numerade.com
SOLVED Write the complete electron configuration for the scandium atom Iron Notation Electron Configuration The initial two electrons in the electron configuration for iron will be in the 1s orbital. To write the electron configuration for iron, use the notation: We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. Each box represents an atomic orbital. Commonly, the electron configuration is used to describe. Iron Notation Electron Configuration.
From quizlet.com
Draw the electron configuration for a neutral atom of iron. Quizlet Iron Notation Electron Configuration Electron configuration chart of all elements is mentioned in the table below. To write the electron configuration for iron, use the notation: Writing the electron configuration, you really only. The electron configuration can be represented using the electrons in boxes notation. The shorthand electron configuration (or noble gas configuration) as well as. We will put all 26 electrons in orbitals. Iron Notation Electron Configuration.
From www.youtube.com
Iron electronic configuration How to Write Iron electronic Iron Notation Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Electron configuration chart of all elements is mentioned in the table below. Each box represents an atomic orbital. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Its valence orbitals are the #4s# and #3d# 's. The complete electron configuration for iron should be written as 1s2 2s2 2p6. Iron Notation Electron Configuration.
From www.sciencefacts.net
Electron Configuration Definition, Examples, Chart, and Diagram Iron Notation Electron Configuration We will put all 26 electrons in orbitals surrounding the nucleus of the iron atom when we write the configuration. To write the electron configuration for iron, use the notation: The shorthand electron configuration (or noble gas configuration) as well as. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. Each box represents an atomic orbital. Its valence orbitals are the #4s#. Iron Notation Electron Configuration.
From mokasinthebig.weebly.com
Iron atomic number mokasinthebig Iron Notation Electron Configuration Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but it can also be used to represent an atom that has ionized into a cation or. To write the electron configuration for iron, use the notation: The initial two electrons in the electron configuration for iron will be in the 1s orbital.. Iron Notation Electron Configuration.
From www.youtube.com
A stepbystep description of how to write the electron configuration Iron Notation Electron Configuration Its core orbitals are the #1s#, #2s#, #2p# 's, #3s#, and #3p# 's. Each box represents an atomic orbital. How many orbitals are required for the electronic configuration of iron? Writing the electron configuration, you really only. 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶. To write the electron configuration for iron, use the notation: How do you write fe. Electron. Iron Notation Electron Configuration.
From ar.inspiredpencil.com
Iron Orbital Notation Iron Notation Electron Configuration How do you write fe. How many orbitals are required for the electronic configuration of iron? To write the electron configuration for iron, use the notation: The initial two electrons in the electron configuration for iron will be in the 1s orbital. Commonly, the electron configuration is used to describe the orbitals of an atom in its ground state, but. Iron Notation Electron Configuration.