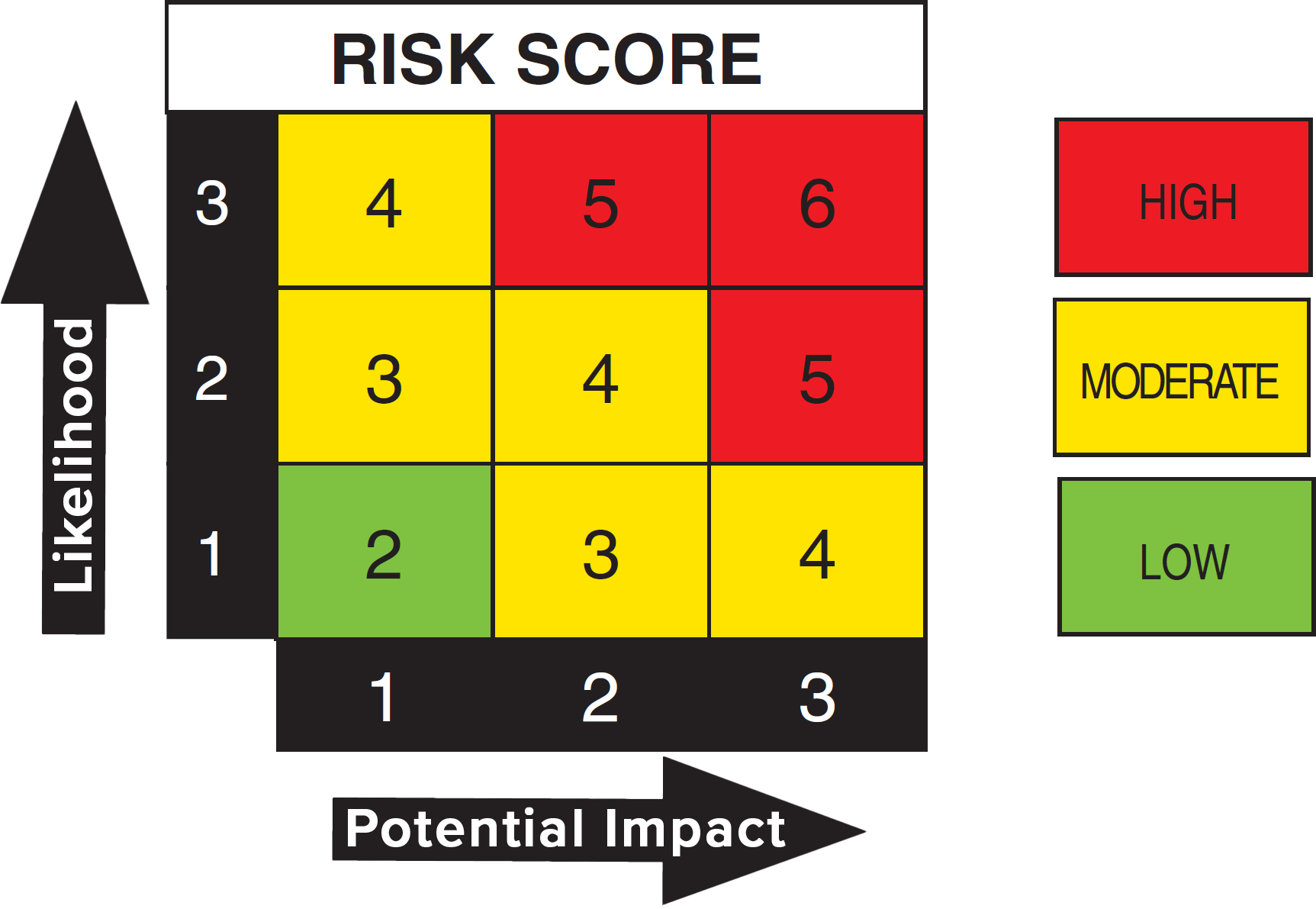
Medical Device Health Hazard Evaluation . Employees, employee representatives, or employers can request an hhe if they have. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is.
from ssaform.com
Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. Employees, employee representatives, or employers can request an hhe if they have. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device.
Hazard Classifications / Risk Assessment FFRP Site Safety Assessment
Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Employees, employee representatives, or employers can request an hhe if they have. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device.
From www.sampletemplates.com
FREE 10+ Sample Health Risk Assessments in PDF MS Word Medical Device Health Hazard Evaluation Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. (1) identification of possible hazards, including [user] error (2). Medical Device Health Hazard Evaluation.
From blog.cm-dm.com
MDR one year left and too late for class I software Software in Medical Device Health Hazard Evaluation A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. Can be useful for determining whether a chemical/compound present or released. Medical Device Health Hazard Evaluation.
From forensicanalytical.com
Reducing Risks in Healthcare Occupations Forensic Analytical Medical Device Health Hazard Evaluation Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. During. Medical Device Health Hazard Evaluation.
From www.researchgate.net
2. List of Hazards considered in the Risk Assessment Download Table Medical Device Health Hazard Evaluation Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. Employees, employee representatives, or employers can request an hhe if they have. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. The notification and evaluation of adverse incidents and field safety. Medical Device Health Hazard Evaluation.
From www.semanticscholar.org
Case study — Risk management for medical devices (based on ISO 14971 Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. A health hazard evaluation (hhe). Medical Device Health Hazard Evaluation.
From www.hotzxgirl.com
Hazard Assessment Form Template And Example Word Doc In Form Hot Sex Medical Device Health Hazard Evaluation Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Employees, employee representatives, or employers can request an. Medical Device Health Hazard Evaluation.
From www.healthleadersmedia.com
Home Medical Devices Tops ECRI's List of Healthcare Technology Hazards Medical Device Health Hazard Evaluation A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. During the process of assessing whether. Medical Device Health Hazard Evaluation.
From breedingbusiness.com
Health & Safety On The Farmyard The Dangers & How To Avoid Them Medical Device Health Hazard Evaluation Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Employees, employee representatives, or employers can request an hhe if they have. A health hazard evaluation (hhe) is an evaluation of possible health hazards. Medical Device Health Hazard Evaluation.
From www.orielstat.com
Medical Device Risk Management Analysis & Tools Oriel STAT A MATRIX Medical Device Health Hazard Evaluation Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health. Medical Device Health Hazard Evaluation.
From aniyah-has-strong.blogspot.com
Special Risk Devices Include Which of the Following AniyahhasStrong Medical Device Health Hazard Evaluation A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Employees, employee representatives, or employers can request an hhe if they have. During the process of assessing whether a correction or removal must take place, a company should be conducting a. Medical Device Health Hazard Evaluation.
From mungfali.com
Hazard Identification Checklist Template Medical Device Health Hazard Evaluation Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. Health. Medical Device Health Hazard Evaluation.
From www.sterlingpharmasolutions.com
Making sense of hazard evaluation Sterling Pharma Solutions Medical Device Health Hazard Evaluation The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Can. Medical Device Health Hazard Evaluation.
From ecampusontario.pressbooks.pub
3.3 Hazard Recognition Canadian Health and Safety Workplace Fundamentals Medical Device Health Hazard Evaluation Employees, employee representatives, or employers can request an hhe if they have. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Annex xv. Medical Device Health Hazard Evaluation.
From www.greenlight.guru
Understanding ISO 14971 Medical Device Risk Management Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. The notification and. Medical Device Health Hazard Evaluation.
From www.webinarcompliance.com
Risk Assessment for Medical Devices inar Compliance Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic. Medical Device Health Hazard Evaluation.
From www.indegene.com
Indegene Accelerates Hazard Evaluation and Risk Analysis Process for Medical Device Health Hazard Evaluation A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine. Medical Device Health Hazard Evaluation.
From www.evaluationforms.org
Health Risk Assessment Online Review Assessment Forms Medical Device Health Hazard Evaluation The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine. Medical Device Health Hazard Evaluation.
From dokumen.tips
(PDF) Health Hazard Evaluation DOKUMEN.TIPS Medical Device Health Hazard Evaluation Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. A health hazard evaluation (hhe) is an evaluation of. Medical Device Health Hazard Evaluation.
From joyfill.io
Fire Sprinkler System Hazard Evaluation Form (NFPA 25) for Mobile Medical Device Health Hazard Evaluation During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is.. Medical Device Health Hazard Evaluation.
From www.templateroller.com
OSHA Form ICS4 Fill Out, Sign Online and Download Printable PDF Medical Device Health Hazard Evaluation Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. During the process of assessing whether a correction or removal must take place, a company should be conducting. Medical Device Health Hazard Evaluation.
From www.orielstat.com
Creating a Medical Device Risk Management Plan and Doing Analysis Medical Device Health Hazard Evaluation During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Can be useful for determining whether a chemical/compound present or released from a medical device presents a. Medical Device Health Hazard Evaluation.
From www.dotcompliance.com
The Essential Guide to ISO 14971 Dot Compliance Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. During. Medical Device Health Hazard Evaluation.
From www.slideserve.com
PPT Medical Device Risk Management Practical Overview & Challenges Medical Device Health Hazard Evaluation The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. Employees, employee representatives, or employers can request an hhe if they have. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. During the process of assessing whether a correction or removal must take. Medical Device Health Hazard Evaluation.
From medicaldevicehq.com
Using MS Excel for medical device risk management Medical Device Health Hazard Evaluation Employees, employee representatives, or employers can request an hhe if they have. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. A health hazard evaluation (hhe) is. Medical Device Health Hazard Evaluation.
From dchas.org
Update on Chemical Safety Information in PubChem ACS Division of Medical Device Health Hazard Evaluation The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical devices is. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard. Medical Device Health Hazard Evaluation.
From tr.linkedin.com
FDA Health Hazard Evaluations and Risk Assesment Medical Device Health Hazard Evaluation During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. Can be useful for determining whether a chemical/compound present or. Medical Device Health Hazard Evaluation.
From www.advena.mt
The Importance of Assessing Medical Device Risks and Hazards for Medical Device Health Hazard Evaluation Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. The notification and evaluation of adverse incidents and field safety corrective actions (fsca) involving medical. Medical Device Health Hazard Evaluation.
From www.industryevents.com
Industry Events Medical Device Hazard Analysis Following ISO 14971 Medical Device Health Hazard Evaluation During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and. Medical Device Health Hazard Evaluation.
From medicaldevicehq.com
FMEA vs ISO 14971 Medical Device HQ 1 Medical Device Health Hazard Evaluation During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine. Medical Device Health Hazard Evaluation.
From mungfali.com
Hazard Identification Checklist Template Medical Device Health Hazard Evaluation Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. Employees, employee representatives, or employers can request an hhe if they have. A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda. Medical Device Health Hazard Evaluation.
From ssaform.com
Hazard Classifications / Risk Assessment FFRP Site Safety Assessment Medical Device Health Hazard Evaluation Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During. Medical Device Health Hazard Evaluation.
From www.healthdigital.uk
Health hazard evaluation medical device Health Digital Medical Device Health Hazard Evaluation A health hazard evaluation (hhe) is an evaluation of possible health hazards at a workplace. Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. The notification and evaluation of adverse incidents and field safety corrective. Medical Device Health Hazard Evaluation.
From www.ismp.org
Gaps in Recalls of HomeUse Medical Devices Top ECRI’s Hazards List for Medical Device Health Hazard Evaluation (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During the process of assessing whether a correction or removal must take place, a company should be conducting a health hazard evaluation (hhe) to determine whether a violation. Employees, employee representatives, or employers can request an hhe if they have. Health hazard evaluations (hhes) and health. Medical Device Health Hazard Evaluation.
From www.riskpal.com
Risk Assessment Matrices Tools to Visualise Risk Medical Device Health Hazard Evaluation Health hazard evaluations (hhes) and health risk assessments (hras) are the processes that fda follows to determine the risks of certain device. Can be useful for determining whether a chemical/compound present or released from a medical device presents a systemic toxic,. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. Annex xv of the (eu). Medical Device Health Hazard Evaluation.
From healthandsafety.com.mt
General Risk Assessment St. Bernard's Health and Safety Institute Medical Device Health Hazard Evaluation Employees, employee representatives, or employers can request an hhe if they have. Annex xv of the (eu) regulations for medical devices 2017/745 (mdr) that a biological safety evaluation needs to be carried out before any. (1) identification of possible hazards, including [user] error (2) risk calculation/estimation, normal and fault. During the process of assessing whether a correction or removal must. Medical Device Health Hazard Evaluation.