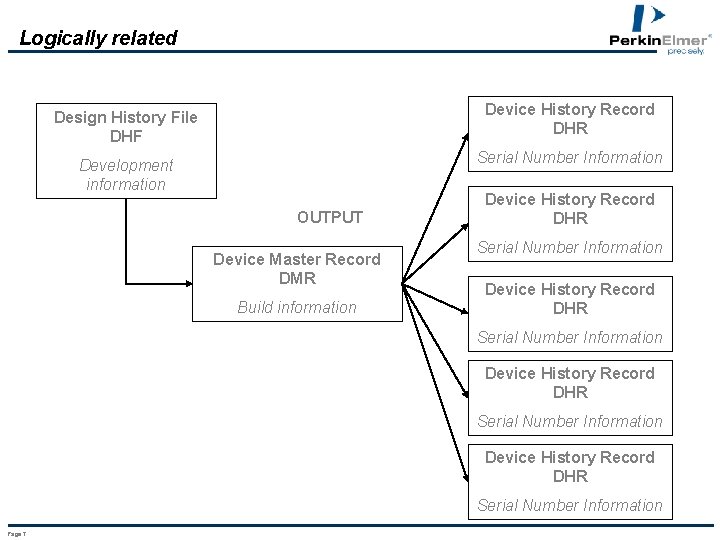
Device Master Record Definition . Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). Describe requirements and intent for. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Each manufacturer shall ensure that each dmr is. What is the device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes. Each manufacturer shall maintain device master records (dmr's). The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. § 820.181 device master record. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. The device master record is a regulatory requirement for all medical device companies. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality.
from slidetodoc.com
Each manufacturer shall ensure that each dmr is. Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. What is the device master record (dmr)? Identify key definitions related to documents and records. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall maintain device master records (dmr's). The device master record is a regulatory requirement for all medical device companies. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality.
Design History File Device Master Record and Device
Device Master Record Definition The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall maintain device master records (dmr's). The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. § 820.181 device master record. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall maintain device master records (dmr's). It is a repository of all essential information about your company’s medical devices and includes. The device master record is a regulatory requirement for all medical device companies. Describe requirements and intent for. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Identify key definitions related to documents and records. Each manufacturer shall ensure that each dmr is. What is the device master record (dmr)?
From www.arenasolutions.com
Managing The Device Master Record (DMR) Arena Device Master Record Definition Describe requirements and intent for. What is the device master record (dmr)? Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that. Device Master Record Definition.
From www.technia.se
What is a Device Master Record? TECHNIA (Sweden) Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). What is the device master record (dmr)? § 820.181 device master record. It is a repository of all essential information about your company’s medical devices and includes. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. The. Device Master Record Definition.
From www.scribd.com
Device Master Records PDF Specification (Technical Standard) Quality Management System Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). It is a repository of all essential information about your company’s medical devices and includes. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall ensure that each dmr is. What is the. Device Master Record Definition.
From www.scribd.com
Device Master Records.doc Specification (Technical Standard) Quality Management System Device Master Record Definition § 820.181 device master record. It is a repository of all essential information about your company’s medical devices and includes. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall ensure that each dmr is. The 21 cfr 820.181 regulation requires all medical device manufacturers to. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID2834106 Device Master Record Definition The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer. Device Master Record Definition.
From www.greenlight.guru
What is Device Master Record (DMR)? Greenlight Guru Device Master Record Definition The device master record is a regulatory requirement for all medical device companies. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. Each manufacturer shall maintain device master records. Device Master Record Definition.
From dokumen.tips
(PDF) DMRDevice Master Record vs DHFDesign History · PDF fileDMRDevice Master Record vs Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall maintain device master records (dmr's). A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a. Device Master Record Definition.
From www.presentationeze.com
Device Master Record (DMR) What needs to be recorded into the DMR.PresentationEZE Device Master Record Definition The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Each manufacturer shall maintain device. Device Master Record Definition.
From aplyonqms.com
Device History Record Procedure A. P. LYON Device Master Record Definition Identify key definitions related to documents and records. Describe requirements and intent for. Each manufacturer shall ensure that each dmr is. It is a repository of all essential information about your company’s medical devices and includes. Each manufacturer shall maintain device master records (dmr's). What is the device master record (dmr)? The device master record (dmr) is focused on building. Device Master Record Definition.
From www.youtube.com
Preparing a Device Master Record (DMR) YouTube Device Master Record Definition A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Describe requirements and intent for. Identify key definitions related to documents and records. Each manufacturer shall ensure that each dmr is. Each manufacturer shall maintain device master records (dmr's). The device master record is a regulatory requirement for all. Device Master Record Definition.
From slidetodoc.com
Design History File Device Master Record and Device Device Master Record Definition What is the device master record (dmr)? It is a repository of all essential information about your company’s medical devices and includes. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. § 820.181 device master record. The device master record is a regulatory requirement for. Device Master Record Definition.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Definition It is a repository of all essential information about your company’s medical devices and includes. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents. Device Master Record Definition.
From www.qualitymeddev.com
Device Master Record Overview of FDA Requiements Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. The device master record (dmr) is focused on building the device and ensuring that all necessary items. Device Master Record Definition.
From www.technia.us
What is a Device Master Record? TECHNIA (US) Device Master Record Definition § 820.181 device master record. What is the device master record (dmr)? Each manufacturer shall ensure that each dmr is. It is a repository of all essential information about your company’s medical devices and includes. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. Describe. Device Master Record Definition.
From www.arenasolutions.com
Device Master Record (DMR) Definition Arena Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). Describe requirements and intent for. It is a repository of all essential information about your company’s medical devices and includes. Each manufacturer shall ensure that each dmr is. Identify key definitions related to documents and records. The design history file (dhf) is focused on capturing the history of the design and ensuring. Device Master Record Definition.
From www.linkedin.com
The Device Master Record DMR Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID2688095 Device Master Record Definition § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. Each manufacturer shall ensure that each dmr is. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete. Device Master Record Definition.
From hardcoreqms.com
Device Master Records (DMR) for Medical Devices (2023) Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. § 820.181 device master record. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall ensure that each dmr. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID1510101 Device Master Record Definition The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. Identify key definitions related to documents and records. Each manufacturer shall maintain device master records (dmr's). A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a. Device Master Record Definition.
From www.bizmanualz.com
Device Master Record Procedure Template Word Device Master Record Definition It is a repository of all essential information about your company’s medical devices and includes. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. A device. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID2688095 Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall maintain device master records. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID1082885 Device Master Record Definition § 820.181 device master record. It is a repository of all essential information about your company’s medical devices and includes. What is the device master record (dmr)? Each manufacturer shall maintain device master records (dmr's). The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. A. Device Master Record Definition.
From www.technia.com
What is a Device Master Record? TECHNIA Device Master Record Definition Describe requirements and intent for. Identify key definitions related to documents and records. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. It is a repository of. Device Master Record Definition.
From www.greenlight.guru
Design History File (DHF) vs. Device Master Record (DMR) vs. Device History Record (DHR Device Master Record Definition What is the device master record (dmr)? The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. Identify key definitions related to documents and records. § 820.181 device master record. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according. Device Master Record Definition.
From www.dvelop-ls.de
Device Master Record (DMR) Erklärung im Glossar lesen Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. Identify key definitions related to documents and records. Each manufacturer shall ensure that each dmr is. Describe requirements and intent for. The device master record is a regulatory requirement for all medical device companies. § 820.181 device master record. The design. Device Master Record Definition.
From www.slideserve.com
PPT Introduction to Quality Management Systems for Medical Devices PowerPoint Presentation Device Master Record Definition A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall maintain device master records (dmr's). Describe requirements and intent for. It is a repository of all essential information about your company’s medical devices and includes. What is the. Device Master Record Definition.
From www.instantgmp.com
Device Master Record Device Master Record Definition Each manufacturer shall ensure that each dmr is. The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Describe requirements and intent for. Identify key definitions related to documents and records. The device master record is a regulatory. Device Master Record Definition.
From studylib.net
Device Master Record Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). § 820.181 device master record. What is the device master record (dmr)? Each manufacturer shall ensure that each dmr is. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done according to fda regulation. Each manufacturer shall maintain device master records (dmr's).. Device Master Record Definition.
From www.slideserve.com
PPT Product Documentation PowerPoint Presentation, free download ID2967112 Device Master Record Definition Each manufacturer shall maintain device master records (dmr's). It is a repository of all essential information about your company’s medical devices and includes. Describe requirements and intent for. Identify key definitions related to documents and records. What is the device master record (dmr)? The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device. Device Master Record Definition.
From bizmanualz.com
Device Master Record Procedure Device Master Record Definition The device master record is a regulatory requirement for all medical device companies. Each manufacturer shall maintain device master records (dmr's). Each manufacturer shall ensure that each dmr is. § 820.181 device master record. The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it.. Device Master Record Definition.
From www.youtube.com
Device Master Record & Device History Record A Regulatory YouTube Device Master Record Definition It is a repository of all essential information about your company’s medical devices and includes. What is the device master record (dmr)? § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). The device master record is a regulatory requirement for all medical device companies. Describe requirements and intent for. The design history file (dhf) is focused. Device Master Record Definition.
From criticalthinking.cloud
what is an device history record Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. The design history file (dhf) is focused on capturing the history of the design and ensuring that it was done. Device Master Record Definition.
From www.technia.co.uk
What is a Device Master Record? TECHNIA (UK) Device Master Record Definition Identify key definitions related to documents and records. The device master record is a regulatory requirement for all medical device companies. § 820.181 device master record. Each manufacturer shall ensure that each dmr is. Each manufacturer shall maintain device master records (dmr's). The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications,. Device Master Record Definition.
From www.slideserve.com
PPT Design Documentation PowerPoint Presentation, free download ID1510101 Device Master Record Definition The 21 cfr 820.181 regulation requires all medical device manufacturers to maintain concrete records related to device specifications, quality. What is the device master record (dmr)? § 820.181 device master record. Each manufacturer shall maintain device master records (dmr's). Identify key definitions related to documents and records. Each manufacturer shall ensure that each dmr is. Describe requirements and intent for.. Device Master Record Definition.
From www.bizmanualz.com
Device Master Record Index Template Device Master Record Definition The device master record (dmr) is focused on building the device and ensuring that all necessary items are included to build, test, package, and service it. A device master record (dmr) is a comprehensive compilation of all the instructions, drawings, and specifications needed to manufacture a specific. Describe requirements and intent for. Each manufacturer shall maintain device master records (dmr's).. Device Master Record Definition.