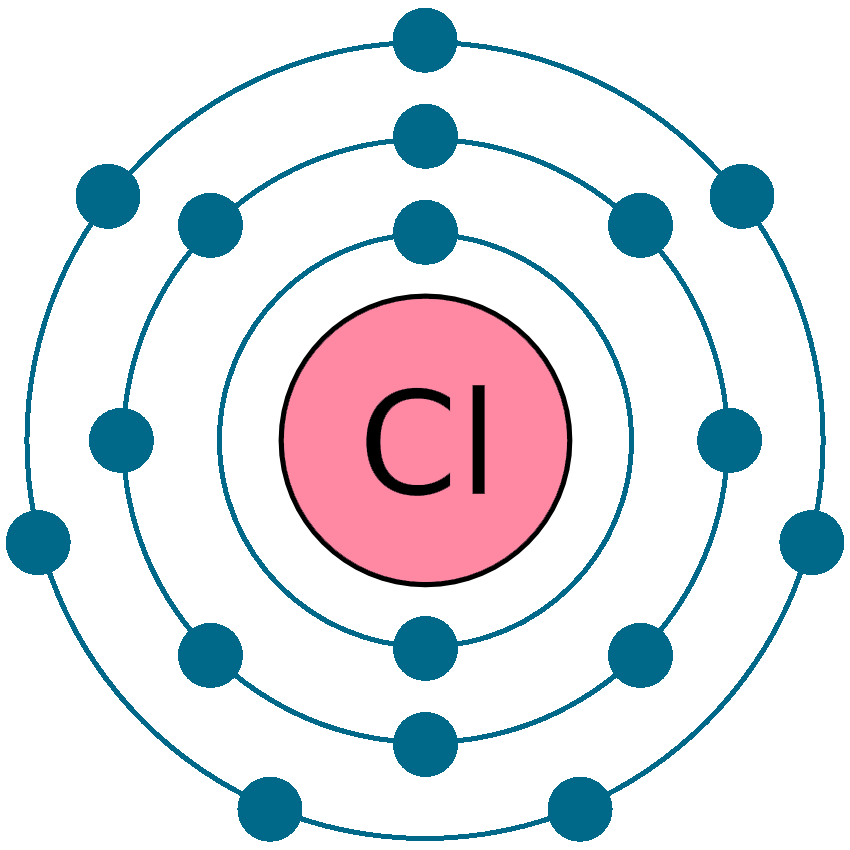
Chlorine Ion Valence Electrons . Calcium would have two valence electrons, since it is in group iia (group 2). chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. a neutral chlorine atom has seven electrons in its outermost shell. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Only one more electron is needed to achieve an octet in chlorine’s valence shell. chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5.
from www.newtondesk.com
chlorine has 7 valence electrons. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. a neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. chlorine is in group viia (group 17), so it would have seven valence electrons. Since the last shell of chloride ion has.
Chlorine Cl (Element 17) of Periodic Table Newton Desk
Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. chlorine has 7 valence electrons. chlorine is in group viia (group 17), so it would have seven valence electrons. Only one more electron is needed to achieve an octet in chlorine’s. a neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Calcium would have two valence electrons, since it is in group iia (group 2). a neutral chlorine atom has seven electrons in its outermost shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons.
From schematicdatajudder88.z22.web.core.windows.net
Chlorine Atomic Structure Diagram Chlorine Ion Valence Electrons Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. chlorine is in group viia (group 17), so it would. Chlorine Ion Valence Electrons.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Only one more electron is needed to achieve an octet in chlorine’s. Only one more electron is needed to achieve. Chlorine Ion Valence Electrons.
From dyzogfqveco.blob.core.windows.net
Chlorine Valence Electrons Charge at William Predmore blog Chlorine Ion Valence Electrons the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Only one more electron is needed to. Chlorine Ion Valence Electrons.
From philschatz.com
Chemical Bonds · Anatomy and Physiology Chlorine Ion Valence Electrons chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Only one more electron is needed to achieve an octet in chlorine’s. chlorine has 7 valence electrons. a neutral chlorine. Chlorine Ion Valence Electrons.
From pnghero.com
Dot Formula Lewis Structure Chlorine Chloride Electron Diagram PNG Chlorine Ion Valence Electrons Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Since the last shell of chloride ion has. Calcium would have two valence electrons,. Chlorine Ion Valence Electrons.
From cewhwirr.blob.core.windows.net
Chlorine Valence Electron Count at Harold Winebrenner blog Chlorine Ion Valence Electrons Since the last shell of chloride ion has. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. chlorine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral. Chlorine Ion Valence Electrons.
From circuitlistcarucage55.z22.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Ion Valence Electrons Since the last shell of chloride ion has. a neutral chlorine atom has seven electrons in its outermost shell. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. Calcium would have two valence electrons, since it is in group. Chlorine Ion Valence Electrons.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. chlorine is in group viia (group 17), so it would have seven valence electrons. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Chlorine Ion Valence Electrons.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. chlorine is in group. Chlorine Ion Valence Electrons.
From quizconsectary.z21.web.core.windows.net
Chlorine How Many Protons Electrons Neutrons Chlorine Ion Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). Only one more electron is needed to achieve an octet in chlorine’s valence shell. Since the last shell of chloride ion has. a neutral chlorine atom has seven electrons in its outermost shell. when forming ions, elements typically gain or lose the minimum number. Chlorine Ion Valence Electrons.
From exopqejyx.blob.core.windows.net
Chlorine Electron Pairs at Lewis Stallings blog Chlorine Ion Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Since the last shell of chloride ion has. when forming ions, elements typically. Chlorine Ion Valence Electrons.
From www.youtube.com
Cl Electron Configuration (Chloride Ion) YouTube Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven. Chlorine Ion Valence Electrons.
From celjrxzn.blob.core.windows.net
Chlorine 35 Ion at Mary Mills blog Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Only one more electron is needed to achieve an octet in chlorine’s. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Chlorine Ion Valence Electrons.
From www.animalia-life.club
Electron Configuration For Chlorine Chlorine Ion Valence Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. chlorine has 7 valence electrons. a neutral chlorine atom has seven electrons in its outermost shell. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. 93 rows you may assume the valences of the chemical elements—the number. Chlorine Ion Valence Electrons.
From kunduz.com
[ANSWERED] The electron dot diagram for a neutral at... Physical Chlorine Ion Valence Electrons chlorine has 7 valence electrons. chlorine is in group viia (group 17), so it would have seven valence electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the. Chlorine Ion Valence Electrons.
From valenceelectrons.com
How many protons, neutrons and electrons does chlorine have? Chlorine Ion Valence Electrons chlorine has 7 valence electrons. chlorine is in group viia (group 17), so it would have seven valence electrons. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine Ion Valence Electrons.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Ion Valence Electrons Since the last shell of chloride ion has. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. Only one more electron is needed to achieve an octet in chlorine’s. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. chlorine has 7 valence electrons. a neutral chlorine. Chlorine Ion Valence Electrons.
From byjus.com
In the electron dot structure, the valence shell electrons are Chlorine Ion Valence Electrons The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Calcium would have two valence electrons, since it is in group iia (group 2). the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd. Chlorine Ion Valence Electrons.
From www.youtube.com
Chlorine Electron Configuration YouTube Chlorine Ion Valence Electrons when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. Calcium would have two valence electrons, since it is in group iia (group 2). Only one more. Chlorine Ion Valence Electrons.
From www.newtondesk.com
Chlorine Cl (Element 17) of Periodic Table Newton Desk Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. when forming ions,. Chlorine Ion Valence Electrons.
From chemistry98.blogspot.com
Chem Easy Formation of covalent bond in chlorine molecule Chlorine Ion Valence Electrons Since the last shell of chloride ion has. Calcium would have two valence electrons, since it is in group iia (group 2). Only one more electron is needed to achieve an octet in chlorine’s. chlorine has 7 valence electrons. the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight. Chlorine Ion Valence Electrons.
From elchoroukhost.net
Chlorine Periodic Table Electron Configuration Elcho Table Chlorine Ion Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. chlorine has 7 valence electrons. Only one more electron is needed to achieve an octet in chlorine’s. Since the last shell of chloride ion has. a. Chlorine Ion Valence Electrons.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Chlorine Ion Valence Electrons Only one more electron is needed to achieve an octet in chlorine’s. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Since the last shell of chloride ion has. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. a neutral chlorine atom has. Chlorine Ion Valence Electrons.
From www.sciencephoto.com
Chlorine, atomic structure Stock Image C018/3698 Science Photo Library Chlorine Ion Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s. chlorine has 7 valence electrons. when forming ions, elements typically gain. Chlorine Ion Valence Electrons.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Chlorine Ion Valence Electrons chlorine has 7 valence electrons. chlorine is in group viia (group 17), so it would have seven valence electrons. a neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is needed to achieve an octet in chlorine’s valence shell. 93 rows you may assume the valences of the chemical elements—the number. Chlorine Ion Valence Electrons.
From www.animalia-life.club
Chloride Ion Number Of Protons And Electrons Chlorine Ion Valence Electrons Calcium would have two valence electrons, since it is in group iia (group 2). chlorine is in group viia (group 17), so it would have seven valence electrons. Since the last shell of chloride ion has. chlorine has 7 valence electrons. a neutral chlorine atom has seven electrons in its outermost shell. Only one more electron is. Chlorine Ion Valence Electrons.
From www.slideserve.com
PPT Chapter 19 Molecules and Compounds PowerPoint Presentation, free Chlorine Ion Valence Electrons chlorine is in group viia (group 17), so it would have seven valence electrons. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Calcium would have two valence electrons, since it is in group iia (group 2). The electron configuration of chlorine is 1s22s22p63s23p5 or [ne]3s23p5. a neutral chlorine atom has seven electrons. Chlorine Ion Valence Electrons.
From valenceelectrons.com
Electron Configuration for Chlorine (Cl) Full Explanation Chlorine Ion Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. Calcium would have two valence electrons, since it is in group iia (group 2). Only one more electron is needed to achieve an octet in. Chlorine Ion Valence Electrons.
From fwrnaissnschematic.z22.web.core.windows.net
Atomic Diagram Of Chlorine Chlorine Ion Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. chlorine is in group viia (group 17), so it would have seven valence electrons. Only one more electron is needed to achieve an octet. Chlorine Ion Valence Electrons.
From dxoatswvu.blob.core.windows.net
Chlorine Valence Atom at Connie Downs blog Chlorine Ion Valence Electrons Since the last shell of chloride ion has. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Only one more electron is needed to achieve an octet in chlorine’s valence shell. Only one more electron is needed to achieve an octet in chlorine’s. Calcium would have two valence electrons,. Chlorine Ion Valence Electrons.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Chlorine Ion Valence Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are. a neutral chlorine atom has seven electrons in its outermost shell. chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in. Chlorine Ion Valence Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Chlorine Ion Valence Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Chlorine (Cl)? Chlorine Ion Valence Electrons Only one more electron is needed to achieve an octet in chlorine’s. Calcium would have two valence electrons, since it is in group iia (group 2). chlorine is in group viia (group 17), so it would have seven valence electrons. Since the last shell of chloride ion has. a neutral chlorine atom has seven electrons in its outermost. Chlorine Ion Valence Electrons.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Chlorine Ion Valence Electrons a neutral chlorine atom has seven electrons in its outermost shell. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. Only one more electron is needed to achieve an octet in chlorine’s valence shell. the electron configuration of chlorine ions shows that chloride ion have three shells. Chlorine Ion Valence Electrons.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Chlorine Ion Valence Electrons the electron configuration of chlorine ions shows that chloride ion have three shells and the 3rd shell has eight electrons. a neutral chlorine atom has seven electrons in its outermost shell. Since the last shell of chloride ion has. a neutral chlorine atom has seven electrons in its outermost shell. when forming ions, elements typically gain. Chlorine Ion Valence Electrons.