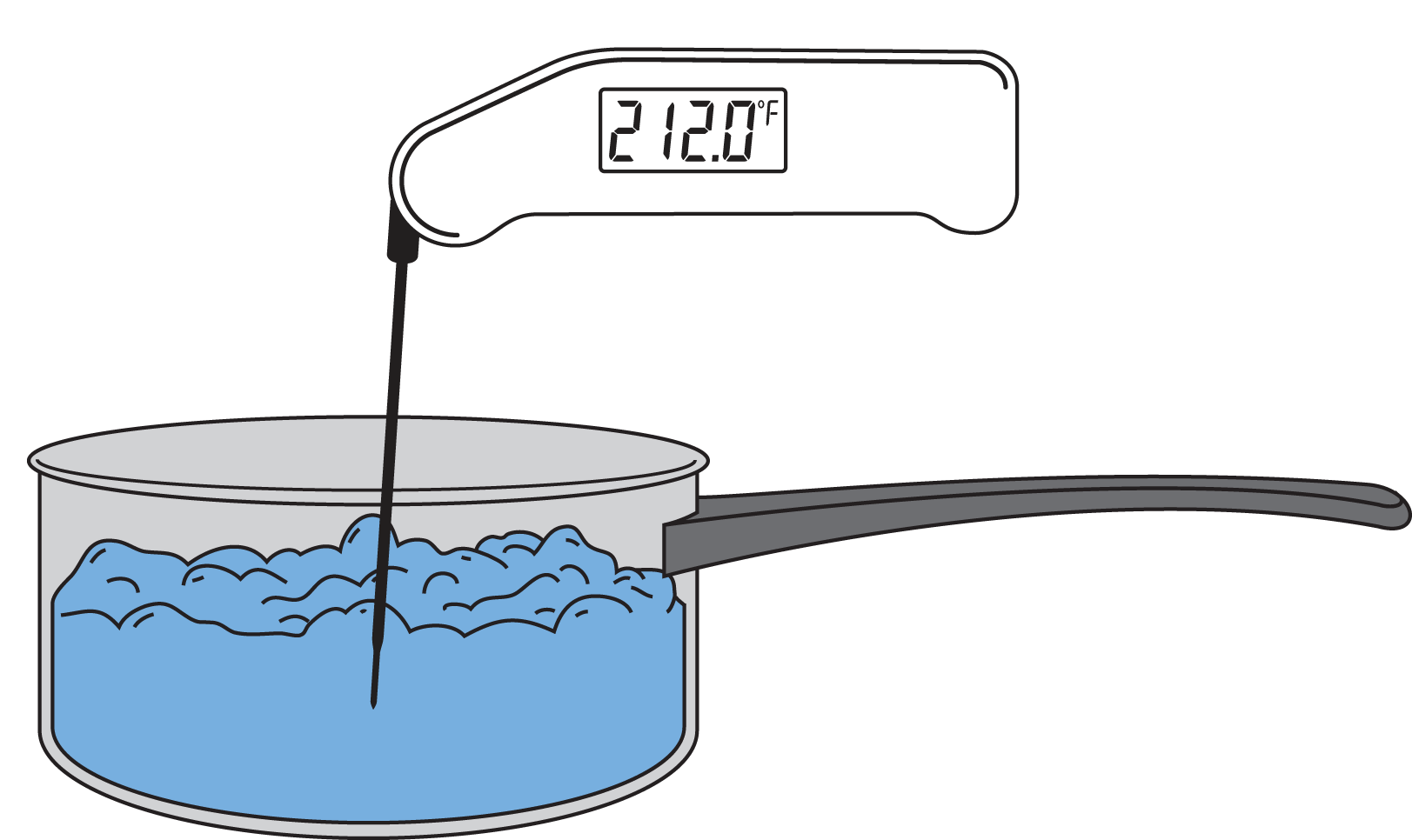
What Temperature Does It Take To Boil Water . At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. That means in most places this is the temperatures. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. At what temperature does water boil? The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level.
from www.thermoworks.com
When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. That means in most places this is the temperatures. At what temperature does water boil? Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid.
Thermal Secrets to Boiling Point Calibration ThermoWorks
What Temperature Does It Take To Boil Water When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. At what temperature does water boil? At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. That means in most places this is the temperatures.
From www.youtube.com
Find how long it takes to bring a cup of water to boiling temperature What Temperature Does It Take To Boil Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. That means in most places this is the temperatures. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point. What Temperature Does It Take To Boil Water.
From ar.inspiredpencil.com
Boiling Point Of Water Examples What Temperature Does It Take To Boil Water Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature. What Temperature Does It Take To Boil Water.
From www.slideserve.com
PPT Freezing/Melting and Boiling Points PowerPoint Presentation, free What Temperature Does It Take To Boil Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea. What Temperature Does It Take To Boil Water.
From www.thermoworks.com
Thermal Secrets to Boiling Point Calibration ThermoWorks What Temperature Does It Take To Boil Water Normally when we boil a liquid, we do so at atmospheric pressure. That means in most places this is the temperatures. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. If. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At what temperature does water boil? When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. That means in most places this is the temperatures. If this pressure is the standard pressure of 1 atm (101.3 kpa),. What Temperature Does It Take To Boil Water.
From waterfiltersadvisor.com
How Long Does it Take to Boil Water? (4 Factors Affecting Boiling Time) What Temperature Does It Take To Boil Water At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. That means in most places this is the temperatures. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water That means in most places this is the temperatures. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point of water is 212 degrees fahrenheit. What Temperature Does It Take To Boil Water.
From www.yiannislucacos.gr
How to boil Boiling as a basic cooking method Yiannis Lucacos What Temperature Does It Take To Boil Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. That means in most places this is the temperatures. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external. What Temperature Does It Take To Boil Water.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Does It Take To Boil Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At sea level,. What Temperature Does It Take To Boil Water.
From hikingmastery.com
Does Boiling Water Purify It Basic Facts and Useful What Temperature Does It Take To Boil Water Normally when we boil a liquid, we do so at atmospheric pressure. At what temperature does water boil? The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. If this pressure is the standard pressure of 1. What Temperature Does It Take To Boil Water.
From worldnewlive.com
At What Temperature Did The Water Start To Boil? Mastery Wiki What Temperature Does It Take To Boil Water Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. That means in most places this is the temperatures. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. The boiling point for water is 212 ºf or 100 ºc, whereas. What Temperature Does It Take To Boil Water.
From apollo.lsc.vsc.edu
Saturation Vapor Pressure and the Boiling Point What Temperature Does It Take To Boil Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. Water boils at a. What Temperature Does It Take To Boil Water.
From www.artofit.org
How long does it take to boil water Artofit What Temperature Does It Take To Boil Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature. What Temperature Does It Take To Boil Water.
From youtube.com
What Temperature does Water Boil? YouTube What Temperature Does It Take To Boil Water At what temperature does water boil? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. That means in most places this is the temperatures. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At sea level, water boils at. What Temperature Does It Take To Boil Water.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Temperature Does It Take To Boil Water That means in most places this is the temperatures. Normally when we boil a liquid, we do so at atmospheric pressure. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At what temperature does water boil? That means in most places this is the temperatures. The boiling point for water is 212. What Temperature Does It Take To Boil Water.
From www.artofit.org
How long does it take to boil water Artofit What Temperature Does It Take To Boil Water At what temperature does water boil? Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Water boils at a lower temperature as. What Temperature Does It Take To Boil Water.
From smartquizregistry.blogspot.com
Smart Quiz Registry At What Temperature Does Water Boil On The Celsius What Temperature Does It Take To Boil Water Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. Normally when we boil a liquid, we do so at atmospheric pressure. That means in most. What Temperature Does It Take To Boil Water.
From cookthink.com
How Long Does it Take for Water to Boil CookThink What Temperature Does It Take To Boil Water At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. Normally when we boil a liquid,. What Temperature Does It Take To Boil Water.
From slideplayer.com
L 19 Thermodynamics [4] Thermal radiation examples Heat capacity What Temperature Does It Take To Boil Water Normally when we boil a liquid, we do so at atmospheric pressure. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At what temperature does water boil? At sea. What Temperature Does It Take To Boil Water.
From sciencenotes.org
How to Boil Water at Room Temperature What Temperature Does It Take To Boil Water When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. That means in most places this is the temperatures. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At what temperature does water boil? The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At sea. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. At what temperature does water boil? If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. When. What Temperature Does It Take To Boil Water.
From www.pinterest.com
How Long Does It Take Water to Boil? Answering the Bubbling Question What Temperature Does It Take To Boil Water Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At what temperature does water boil? If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. When the vapour pressure reaches an equivalent. What Temperature Does It Take To Boil Water.
From www.pinterest.com
What temperature does water boils at? boilingpoint water bp What Temperature Does It Take To Boil Water That means in most places this is the temperatures. At what temperature does water boil? When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. The boiling point for water is 212. What Temperature Does It Take To Boil Water.
From thecookwaregeek.com
How Long Does it Take to Boil Water? The Cookware Geek What Temperature Does It Take To Boil Water When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At sea level, water boils at 100°c (212°f), which is the temperature at which its vapor pressure equals the external pressure. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. Water boils at a lower temperature. What Temperature Does It Take To Boil Water.
From sciencenotes.org
Boiling Point Definition, Temperature, and Examples What Temperature Does It Take To Boil Water If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. The. What Temperature Does It Take To Boil Water.
From worldwaterforum7.org
How Long Does It Take For Water To Boil At Different Temperatures What Temperature Does It Take To Boil Water The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. That means in most places this is the temperatures. At what temperature does. What Temperature Does It Take To Boil Water.
From beezzly.com
How Long Does It Take to Boil Water? Detailed Guide Beezzly What Temperature Does It Take To Boil Water At what temperature does water boil? If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Normally when we boil a liquid, we do so at atmospheric pressure. The. What Temperature Does It Take To Boil Water.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Temperature Does It Take To Boil Water That means in most places this is the temperatures. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. At. What Temperature Does It Take To Boil Water.
From vandewaterbooks.com
What Temperature Does Water Boil At? Boiling Point & Elevation Vande What Temperature Does It Take To Boil Water At what temperature does water boil? Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. That means in most places this is the temperatures. When the vapour pressure reaches an. What Temperature Does It Take To Boil Water.
From cookthink.com
How Long Does it Take for Water to Boil CookThink What Temperature Does It Take To Boil Water The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. Normally when we boil a liquid, we do so at atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid. When the vapour pressure reaches an equivalent. What Temperature Does It Take To Boil Water.
From recipepes.com
what temperature does water boil What Temperature Does It Take To Boil Water Water boils at a lower temperature as you gain altitude (e.g., going higher on a mountain) and boils at a higher temperature if. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. When the vapour pressure reaches an equivalent value to the surrounding air pressure, the liquid will boil. At what temperature does. What Temperature Does It Take To Boil Water.
From www.compoundchem.com
What Temperature Does Water Boil At? Boiling Point & Elevation What Temperature Does It Take To Boil Water At what temperature does water boil? That means in most places this is the temperatures. The boiling point for water is 212 ºf or 100 ºc, whereas the boiling point of salt water is about 102 ºc. The boiling point of water is 212 degrees fahrenheit or 100 degrees celsius at sea level. Normally when we boil a liquid, we. What Temperature Does It Take To Boil Water.