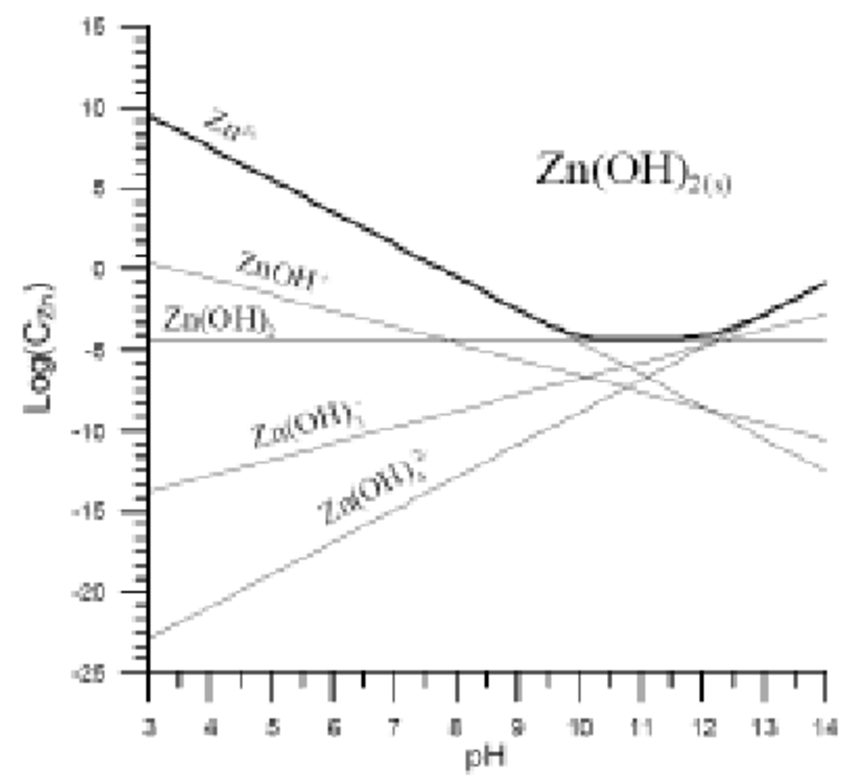
Zinc Ion Solubility In Water . Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. These soluble ones actually react with the water that. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc hydroxide dissolves in aqueous ammonia to form a.
from bceweb.org
Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc hydroxide dissolves in aqueous ammonia to form a. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. It is sparingly soluble in water and absolutely insoluble in alcohol. These soluble ones actually react with the water that. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq).
Zinc Solubility Chart A Visual Reference of Charts Chart Master
Zinc Ion Solubility In Water Zinc hydroxide dissolves in aqueous ammonia to form a. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Examples of solubility of zinc compounds are:. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. These soluble ones actually react with the water that. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc hydroxide dissolves in aqueous ammonia to form a. Anionic znco 3 has a solubility of 0.21 g/l. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and.
From www.flinnsci.ca
Solubility Rules Charts for Chemistry Zinc Ion Solubility In Water These soluble ones actually react with the water that. Examples of solubility of zinc compounds are:. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). All oxides are insoluble except those containing calcium, barium, or alkali metal ions. It is sparingly soluble in water and absolutely insoluble in alcohol. When ionic compounds dissolve in water, the ions. Zinc Ion Solubility In Water.
From socratic.org
How does zinc form an insoluble zinc hydroxide in water? Socratic Zinc Ion Solubility In Water Examples of solubility of zinc compounds are:. It is sparingly soluble in water and absolutely insoluble in alcohol. Anionic znco 3 has a solubility of 0.21 g/l. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Zinc Ion Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility In Water Examples of solubility of zinc compounds are:. Anionic znco 3 has a solubility of 0.21 g/l. It is sparingly soluble in water and absolutely insoluble in alcohol. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc chloride and sulfate are soluble in water, while the. Zinc Ion Solubility In Water.
From mungfali.com
Solubility Rules Flowchart Chart Chemistry Zinc Ion Solubility In Water It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Anionic znco 3 has a solubility of 0.21 g/l. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility of zinc compounds are:. Zinc chloride and sulfate are soluble in water,. Zinc Ion Solubility In Water.
From advancedchemsys.com
Zinc Removal From Water Advanced Chemical Systems Zinc Ion Solubility In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility. Zinc Ion Solubility In Water.
From www.numerade.com
SOLVED a Zn(CN) 2, zinc cyanide Ksp 8.0 X 1012 Molar solubility mlL Zinc Ion Solubility In Water Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc hydroxide dissolves in aqueous ammonia to form a. Anionic znco 3 has a solubility of 0.21 g/l. It is. Zinc Ion Solubility In Water.
From www.slideserve.com
PPT Zn Solubility PowerPoint Presentation, free download ID3226993 Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc hydroxide dissolves in aqueous ammonia to form a. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Examples of solubility of zinc compounds are:. Zinc chloride. Zinc Ion Solubility In Water.
From eureka.patsnap.com
Absolutely watersoluble zinc ion fluorescent probe as well as Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Anionic znco 3 has a solubility of 0.21 g/l. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for. Zinc Ion Solubility In Water.
From www.researchgate.net
Effects of added glycine, alanine and serine on zinc citrate solubility Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Anionic znco 3 has a solubility of 0.21 g/l. These soluble ones actually react with the water that. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and.. Zinc Ion Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). These soluble ones actually react with the water that. Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. Assuming six water molecules (hexaaquo complex) bound to a central. Zinc Ion Solubility In Water.
From www.researchgate.net
Zinc solubility stability of LDRLEzinc chelate, zinc sulphate and zinc Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc hydroxide dissolves in aqueous ammonia to form a. Examples of solubility of zinc compounds are:. Anionic znco 3 has a solubility of 0.21 g/l. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc chloride and sulfate are soluble in water, while the oxide,. Zinc Ion Solubility In Water.
From www.youtube.com
Equation for ZnSO4 + H2O (Zinc sulfate + Water) YouTube Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility of zinc compounds are:. Zinc hydroxide dissolves in aqueous ammonia to form a. It is sparingly soluble in water and absolutely insoluble in alcohol. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Zinc Ion Solubility In Water.
From www.chegg.com
Solved What is the solubility of zinc chlorate in water at Zinc Ion Solubility In Water Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). It is sparingly soluble in water and absolutely. Zinc Ion Solubility In Water.
From woelen.homescience.net
Science made alive Chemistry/Solutions Zinc Ion Solubility In Water It is sparingly soluble in water and absolutely insoluble in alcohol. Examples of solubility of zinc compounds are:. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc hydroxide dissolves in aqueous ammonia to form. Zinc Ion Solubility In Water.
From www.youtube.com
Solubility equilibrium Ksp zinc hydroxide YouTube Zinc Ion Solubility In Water Anionic znco 3 has a solubility of 0.21 g/l. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Examples of solubility of zinc compounds are:. Assuming six water molecules (hexaaquo complex) bound to a. Zinc Ion Solubility In Water.
From www.toppr.com
The solubility product of Zn(OH)2 is 10 at 25^∘C . What would be the Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc dissolves in water as znoh +. Zinc Ion Solubility In Water.
From www.researchgate.net
Solubility diagram of manganese, zinc, and citrate ions in aqueous Zinc Ion Solubility In Water It is sparingly soluble in water and absolutely insoluble in alcohol. Examples of solubility of zinc compounds are:. Zinc hydroxide dissolves in aqueous ammonia to form a. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Anionic znco 3 has a solubility of 0.21 g/l. When ionic compounds. Zinc Ion Solubility In Water.
From www.slideserve.com
PPT Zincite Properties . Growth methods . Applications . PowerPoint Zinc Ion Solubility In Water Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Zinc hydroxide dissolves in aqueous ammonia to form a. It is sparingly soluble in water and absolutely insoluble in alcohol. All oxides are insoluble except those containing. Zinc Ion Solubility In Water.
From www.slideserve.com
PPT Zn Solubility PowerPoint Presentation, free download ID370438 Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility of zinc compounds are:. Zinc hydroxide dissolves in aqueous ammonia to form a. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc chloride and sulfate are soluble in water,. Zinc Ion Solubility In Water.
From www.researchgate.net
Predicted solubility of Al 3+ , Zn 2+ , and Mg 2+ in 0.01 mol dm À3 H 3 Zinc Ion Solubility In Water Examples of solubility of zinc compounds are:. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). When ionic compounds dissolve in water, the. Zinc Ion Solubility In Water.
From www.numerade.com
SOLVED Consider the insoluble compound zinc hydroxide, Zn(OH)2. The Zinc Ion Solubility In Water These soluble ones actually react with the water that. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Examples of solubility of zinc compounds are:. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc chloride and sulfate are soluble in water, while the. Zinc Ion Solubility In Water.
From slidetodoc.com
Solubility Equilibrium The Solubility Product Constant Most salts Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Anionic znco 3 has a solubility of 0.21 g/l. Examples of solubility of zinc compounds are:. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq).. Zinc Ion Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Ion Solubility In Water All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide,. Zinc Ion Solubility In Water.
From eureka.patsnap.com
Absolutely watersoluble zinc ion fluorescent probe as well as Zinc Ion Solubility In Water When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc hydroxide dissolves in aqueous ammonia to form a. These soluble ones actually react with the water that. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. It is sparingly soluble in water. Zinc Ion Solubility In Water.
From www.researchgate.net
Zn 2+ solubility diagram simulated by DIASTAB. Download Scientific Zinc Ion Solubility In Water Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Anionic znco 3 has a solubility of 0.21 g/l. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Zinc hydroxide dissolves. Zinc Ion Solubility In Water.
From www.researchgate.net
Speciation diagram of the zinc complex ions. Download Scientific Diagram Zinc Ion Solubility In Water Zinc hydroxide dissolves in aqueous ammonia to form a. It is sparingly soluble in water and absolutely insoluble in alcohol. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. These soluble ones actually react with the water that. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq).. Zinc Ion Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc carbonate in each of Zinc Ion Solubility In Water These soluble ones actually react with the water that. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Zinc hydroxide dissolves in aqueous ammonia to form a. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. It is sparingly soluble in water and absolutely. Zinc Ion Solubility In Water.
From www.youtube.com
Is Zn(OH)2 Soluble or Insoluble in Water? YouTube Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. These soluble ones actually react with the water that. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Zinc Ion Solubility In Water.
From www.chegg.com
Solved Compare the solubility of zinc sulfide in each of the Zinc Ion Solubility In Water These soluble ones actually react with the water that. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. Anionic znco 3 has a solubility of 0.21 g/l. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water.. Zinc Ion Solubility In Water.
From rayb78.github.io
Solubility In Water Chart Zinc Ion Solubility In Water Examples of solubility of zinc compounds are:. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and. It is sparingly soluble in water and absolutely insoluble in alcohol. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Assuming six water molecules (hexaaquo complex) bound. Zinc Ion Solubility In Water.
From www.youtube.com
Equation for Zn(NO3)2 + H2O (Zinc nitrate + Water) YouTube Zinc Ion Solubility In Water Anionic znco 3 has a solubility of 0.21 g/l. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility of zinc compounds are:. Zinc hydroxide dissolves in aqueous ammonia to form a. These soluble ones actually react with the water that. It is sparingly soluble in water and absolutely insoluble in alcohol. When ionic. Zinc Ion Solubility In Water.
From bceweb.org
Zinc Solubility Chart A Visual Reference of Charts Chart Master Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. All oxides are insoluble except those containing calcium, barium, or alkali metal ions. Examples of solubility of zinc compounds are:.. Zinc Ion Solubility In Water.
From www.numerade.com
SOLVED Consider the insoluble compound zinc sulfide, ZnS. The zinc ion Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water. Zinc Ion Solubility In Water.
From www.researchgate.net
Simplified configuration of zinc reactions with water Download Zinc Ion Solubility In Water Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Zinc dissolves in water as znoh + (aq) or zn 2+ (aq). Zinc chloride and sulfate are soluble in water, while the oxide, sulfide, carbonate, phosphate, silicate, and organic complexes are insoluble or. Examples of solubility of zinc compounds are:. Zinc. Zinc Ion Solubility In Water.
From www.researchgate.net
Solubility diagram for zinc oxide Download Scientific Diagram Zinc Ion Solubility In Water It is sparingly soluble in water and absolutely insoluble in alcohol. Examples of solubility of zinc compounds are:. Zinc hydroxide dissolves in aqueous ammonia to form a. Assuming six water molecules (hexaaquo complex) bound to a central zinc ion, the enthalpy of solvation for one water. Anionic znco 3 has a solubility of 0.21 g/l. Zinc chloride and sulfate are. Zinc Ion Solubility In Water.