Chlorine Has A Higher Ionization Energy Than Aluminum . The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). This is the energy per mole necessary to remove electrons. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy, second ionization energy as well as. As could be expected from their electron configuration, the. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. First ionization energy of chlorine is 12.9676 ev. 120 rows ionization energy chart of all the elements is given below.
from www.numerade.com
120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. First ionization energy of chlorine is 12.9676 ev. This is the energy per mole necessary to remove electrons. These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. As could be expected from their electron configuration, the. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make.
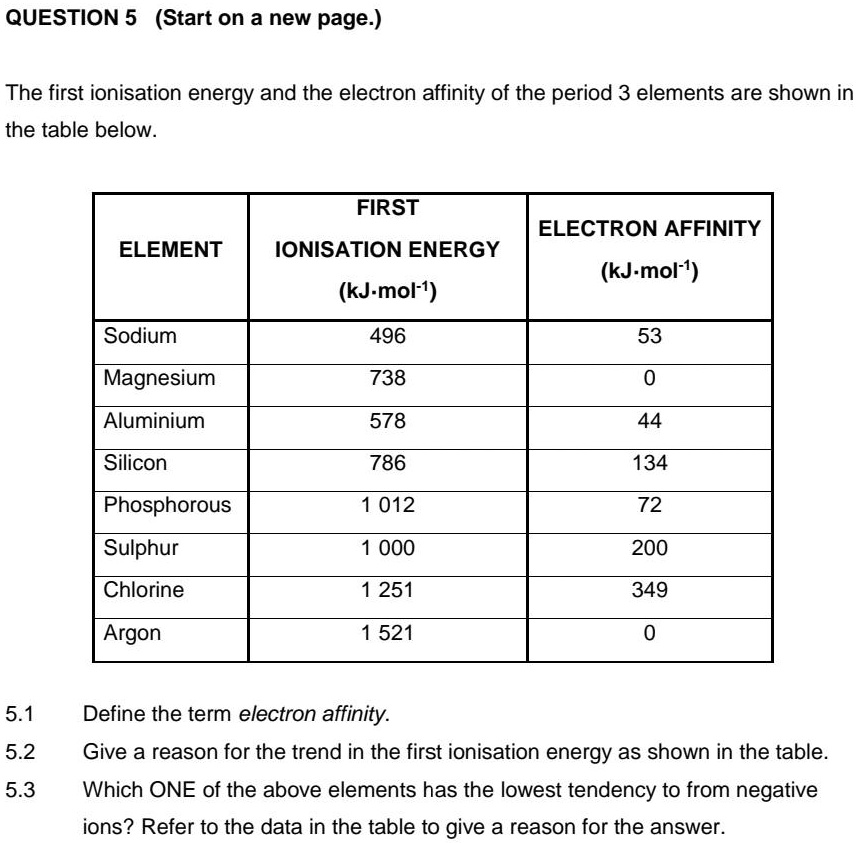
SOLVED QUESTION 5 (Start on a new page. The first ionisation energy
Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy of chlorine is 12.9676 ev. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). First ionization energy, second ionization energy as well as. These tables list values of molar ionization energies, measured in kj⋅mol −1. As could be expected from their electron configuration, the. This is the energy per mole necessary to remove electrons.
From www.numerade.com
SOLVEDUse electron configurations to explain why (a) sulfur has a Chlorine Has A Higher Ionization Energy Than Aluminum These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. First ionization energy of chlorine is 12.9676 ev. As could be expected from their electron configuration, the. This is the energy per mole necessary to remove electrons. Ionization energies of the elements in the third row of the periodic. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.youtube.com
Why is the second ionization energy of alkali metals higher than 1st Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): As could. Chlorine Has A Higher Ionization Energy Than Aluminum.
From slideplayer.com
Trends in ionization energy ppt download Chlorine Has A Higher Ionization Energy Than Aluminum These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy of chlorine is 12.9676 ev. First ionization energy, second ionization energy as well as. This is the energy per mole necessary to remove electrons. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. Ionization energies of the. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.toppr.com
Which of the following statements is incorrect? The second ionization Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy of chlorine is 12.9676 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. 120 rows ionization energy chart of all the elements is given below. This is the energy per mole necessary to remove electrons. The amount of energy required to remove the most loosely bound electron from. Chlorine Has A Higher Ionization Energy Than Aluminum.
From therealgroupichem.weebly.com
Ionization energy Group i Chemistry(iiiescuro) Chlorine Has A Higher Ionization Energy Than Aluminum This is the energy per mole necessary to remove electrons. These tables list values of molar ionization energies, measured in kj⋅mol −1. As could be expected from their electron configuration, the. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). In. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.chegg.com
Solved Argon has a higher ionization energy than chlorine. Chlorine Has A Higher Ionization Energy Than Aluminum These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.chegg.com
Solved 48. Aluminum has a smaller first ionization energy Chlorine Has A Higher Ionization Energy Than Aluminum As could be expected from their electron configuration, the. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. First ionization energy, second ionization energy as well as. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. This. Chlorine Has A Higher Ionization Energy Than Aluminum.
From askfilo.com
The ionization energy of boron is less than that of beryllium because Chlorine Has A Higher Ionization Energy Than Aluminum Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): This is the energy per mole necessary to remove electrons. 120 rows ionization energy chart of all the. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.toppr.com
Which of the following statements is incorrect? The second ionization Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): First ionization energy of chlorine is 12.9676 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from. Chlorine Has A Higher Ionization Energy Than Aluminum.
From askfilo.com
The first ionisation energy of chlorine is greater than that of sulfur.W.. Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. This is the energy per mole necessary to remove electrons. As could be expected. Chlorine Has A Higher Ionization Energy Than Aluminum.
From ben-jolpbloghunter.blogspot.com
Why Does Fluorine Have a High Ionization Energy Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy of chlorine is 12.9676 ev. As could be expected from their electron configuration, the. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. This is the energy per mole necessary to remove electrons. First ionization energy, second ionization energy as well as. 120 rows ionization energy. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.nuclear-power.com
Chlorine Electron Affinity Electronegativity Ionization Energy of Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). As could be expected from their electron configuration, the. This is the energy per mole necessary to remove electrons. First. Chlorine Has A Higher Ionization Energy Than Aluminum.
From slideplayer.com
PERIODICITY Have you ever noticed that people in the same family have Chlorine Has A Higher Ionization Energy Than Aluminum In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy of chlorine is 12.9676 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. Ionization energies of the elements in the third row of the periodic table exhibit the. Chlorine Has A Higher Ionization Energy Than Aluminum.
From neetlab.com
Ionization Enthalpy NEET Lab Chlorine Has A Higher Ionization Energy Than Aluminum As could be expected from their electron configuration, the. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. 120 rows ionization energy chart of. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.numerade.com
SOLVED Arrange these elements according to first ionization energy Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. First ionization energy of chlorine is 12.9676 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. These tables list values of molar ionization energies, measured in kj⋅mol −1. The amount of energy required to remove the most loosely bound electron from a. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.numerade.com
SOLVEDChlorine has a very large first ionization energy, yet it forms Chlorine Has A Higher Ionization Energy Than Aluminum This is the energy per mole necessary to remove electrons. First ionization energy of chlorine is 12.9676 ev. First ionization energy, second ionization energy as well as. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of. Chlorine Has A Higher Ionization Energy Than Aluminum.
From infinitylearn.com
Ionization Enthalpy Factors, Formula & Measurement Principle Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. As could be expected from their electron configuration, the. These tables list values of molar ionization energies, measured in kj⋅mol −1. The amount of energy required to remove the most loosely. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.numerade.com
SOLVEDThe first ionisation energy of aluminium is slightly lower than Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy of chlorine is 12.9676 ev. First ionization energy, second ionization energy as well as. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. As could be expected from their electron configuration, the. These tables list values of molar ionization energies, measured in kj⋅mol −1. Ionization energies of the elements. Chlorine Has A Higher Ionization Energy Than Aluminum.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Aluminum As could be expected from their electron configuration, the. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): First ionization energy of chlorine is 12.9676 ev. First ionization energy, second ionization energy as well as. Ionization energy, also called ionization potential, is the energy. Chlorine Has A Higher Ionization Energy Than Aluminum.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Aluminum These tables list values of molar ionization energies, measured in kj⋅mol −1. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. This is the energy per mole necessary to remove electrons. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.numerade.com
SOLVED The first ionisation energy of aluminium is slightly lower than Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. First ionization energy of chlorine is 12.9676 ev. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). This is the energy per mole necessary to remove electrons. Ionization energies. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.numerade.com
SOLVED QUESTION 5 (Start on a new page. The first ionisation energy Chlorine Has A Higher Ionization Energy Than Aluminum The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). This is the energy per mole necessary to remove electrons. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and. Chlorine Has A Higher Ionization Energy Than Aluminum.
From exopnbdff.blob.core.windows.net
Does Chlorine Have A Higher Ionization Energy Than Aluminum at Beth Chlorine Has A Higher Ionization Energy Than Aluminum Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. First ionization energy, second ionization energy as well as. This is the energy per mole. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.meritnation.com
Why is aluminium first ionization energy less than that of silicon Chlorine Has A Higher Ionization Energy Than Aluminum This is the energy per mole necessary to remove electrons. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various atoms make. As could be expected from their electron configuration, the. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is. Chlorine Has A Higher Ionization Energy Than Aluminum.
From amiah-jolpblogchavez.blogspot.com
Chlorine Has a Higher Ionization Energy Than Aluminum Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. First ionization energy, second ionization energy. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.chegg.com
Solved E) chlorine has a greater ionization energy than Chlorine Has A Higher Ionization Energy Than Aluminum The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). This is the energy per mole necessary to remove electrons. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy, second ionization energy as well as. Ionization. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.periodic-table.org
Chlorine Ionization Energy Chlorine Has A Higher Ionization Energy Than Aluminum As could be expected from their electron configuration, the. 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): The amount of energy required to remove the most loosely bound electron from a. Chlorine Has A Higher Ionization Energy Than Aluminum.
From byjus.com
Which has high ionization energy oxygen or chlorine?? Chlorine Has A Higher Ionization Energy Than Aluminum 120 rows ionization energy chart of all the elements is given below. As could be expected from their electron configuration, the. The amount of energy required to remove the most loosely bound electron from a gaseous atom in its ground state is called its first ionization energy (ie 1). Ionization energy, also called ionization potential, is the energy necessary to. Chlorine Has A Higher Ionization Energy Than Aluminum.
From socratic.org
Why is the first ionization energy of a nonmetal significantly higher Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. As could be expected from their electron configuration, the. These tables list values of molar ionization energies, measured in kj⋅mol −1. 120 rows ionization energy chart of all the elements is given below. In a chemical reaction, understanding ionization energy is important in order to understand the behavior of whether various. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.toppr.com
viii. Crystal radius is larger than covalent radius. ix. Electron Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy of chlorine is 12.9676 ev. This is the energy per mole necessary to remove electrons. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\). Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.ck12.org
Periodic Trends in Ionization Energy CK12 Foundation Chlorine Has A Higher Ionization Energy Than Aluminum As could be expected from their electron configuration, the. First ionization energy, second ionization energy as well as. These tables list values of molar ionization energies, measured in kj⋅mol −1. First ionization energy of chlorine is 12.9676 ev. 120 rows ionization energy chart of all the elements is given below. Ionization energies of the elements in the third row of. Chlorine Has A Higher Ionization Energy Than Aluminum.
From slideplayer.com
Trends in Periodic Table ppt download Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy, second ionization energy as well as. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): This is the energy per mole necessary to remove electrons. The amount of energy required to remove the most loosely bound electron from a gaseous atom. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.youtube.com
Q. Why nitrogen has higher ionisation enthalpy than oxygen? (Class 11 Chlorine Has A Higher Ionization Energy Than Aluminum Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. First ionization energy of chlorine is 12.9676 ev. First ionization energy, second ionization energy as well as. These tables list values of molar ionization energies, measured in kj⋅mol −1. This is the energy per mole necessary to remove electrons. The amount of energy. Chlorine Has A Higher Ionization Energy Than Aluminum.
From www.emaze.com
Untitled at emaze Presentation Chlorine Has A Higher Ionization Energy Than Aluminum Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. First ionization energy, second ionization energy as well as. First ionization energy of chlorine is 12.9676 ev. These. Chlorine Has A Higher Ionization Energy Than Aluminum.
From general.chemistrysteps.com
Ionization energy Chemistry Steps Chlorine Has A Higher Ionization Energy Than Aluminum First ionization energy of chlorine is 12.9676 ev. Ionization energy, also called ionization potential, is the energy necessary to remove an electron from the neutral. Ionization energies of the elements in the third row of the periodic table exhibit the same pattern as those of \(li\) and \(be\) (table \(\pageindex{2}\)): 120 rows ionization energy chart of all the elements is. Chlorine Has A Higher Ionization Energy Than Aluminum.