Fda Otc Medical Device Labeling . Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. In general, labeling for otc medical devices should.
from medicaldevicelicense.com
In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. This action also will make it. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling.
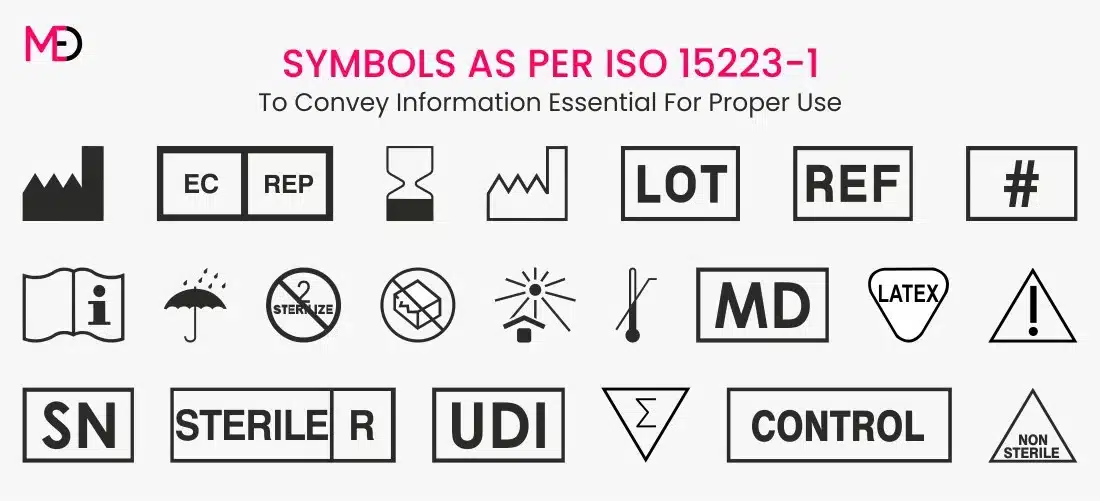
Essential Medical Device Symbols for Labeling ISO 152231
Fda Otc Medical Device Labeling Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices.
From www.tailoredlabel.com
UDI Label Requirements For FDA Medical Device Labels TLP Fda Otc Medical Device Labeling (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In general, labeling for otc medical devices should. The fda’s regulations in 21. Fda Otc Medical Device Labeling.
From www.researchandmarkets.com
US FDA Labeling Requirements for Medical Devices Fda Otc Medical Device Labeling This action also will make it. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part. Fda Otc Medical Device Labeling.
From www.fda.gov
OTC Drug Facts Label FDA Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT Chapter 16 OvertheCounter (OTC) and Prescription Drugs Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Fda has finalized its regulation. Fda Otc Medical Device Labeling.
From www.regdesk.co
FDA on General Principles of Labeling for Medical Devices RegDesk Fda Otc Medical Device Labeling This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In general, labeling for otc medical devices should. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. The fda’s regulations in 21 cfr part. Fda Otc Medical Device Labeling.
From www.greenlight.guru
FDA Medical Device Labeling Checklist [Free Download] Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this. Fda Otc Medical Device Labeling.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Fda Otc Medical Device Labeling Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. This action also will make it. (1) the label of every medical device shall bear a unique device identifier (udi). Fda Otc Medical Device Labeling.
From slideplayer.com
Pilots and Medications ppt download Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). This action also will make. Fda Otc Medical Device Labeling.
From mungfali.com
FDA Medical Device Label Symbols Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical. Fda Otc Medical Device Labeling.
From www.greenlight.guru
FDA Medical Device Labeling Requirements An Overview Fda Otc Medical Device Labeling This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple. Fda Otc Medical Device Labeling.
From vivafda.com
FDA Drug Labeling and Ingredient Requirement Viva FDA U.S. FDA Fda Otc Medical Device Labeling Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart. Fda Otc Medical Device Labeling.
From globalregulatorypartners.com
USFDA Publishes New Requirements for OTC Drugs Labeling Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Fda Otc Medical Device Labeling.
From www.fda.gov
The OvertheCounter Medicine Label Take a Look FDA Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr. Fda Otc Medical Device Labeling.
From vivafda.com
FDA Medical Device Labeling Requirements Viva FDA U.S. FDA Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling.. Fda Otc Medical Device Labeling.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. In general, labeling for otc medical devices should. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Fda Otc Medical Device Labeling.
From mavink.com
Medical Device Labeling Symbols Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set. Fda Otc Medical Device Labeling.
From medicaldevicelicense.com
Essential Medical Device Symbols for Labeling ISO 152231 Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth. Fda Otc Medical Device Labeling.
From exogphupj.blob.core.windows.net
Medical Device Labelling Tga at William Maurer blog Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Fda Otc Medical Device Labeling.
From www.nicelabel.com
FDA UDI compliant labelling NiceLabel Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT HeartStart Home OTC Defibrillator FDA Panel Presentation Fda Otc Medical Device Labeling This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In general, labeling for otc medical devices should. Fda has finalized its regulation that. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT FDA Medical Device Quality System Introduction PowerPoint Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the. Fda Otc Medical Device Labeling.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Fda Otc Medical Device Labeling (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. The fda’s regulations in 21 cfr part 801, subpart. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT Chapter 3 PowerPoint Presentation, free download ID1950891 Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). In general, labeling. Fda Otc Medical Device Labeling.
From www.regdesk.co
FDA Guidance on General Device Labeling RegDesk Fda Otc Medical Device Labeling This action also will make it. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. The fda’s regulations in 21 cfr part 801, subpart c, set forth. Fda Otc Medical Device Labeling.
From www.meddeviceonline.com
Medical Device Labeling New ISO 152231 FDA Guidance UDI Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has. Fda Otc Medical Device Labeling.
From alysidia.com
21 CFR Part 801 FDA Labeling Requirements for Medical Devices Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. In general, labeling for otc medical devices should. This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part. Fda Otc Medical Device Labeling.
From instrktiv.com
IFU for Medical Devices, a Definitive Guide (EU & US) Fda Otc Medical Device Labeling The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi). Fda Otc Medical Device Labeling.
From www.presentationeze.com
FDA Medical Device Classification. PresentationEZE Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices.. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Otc Medical Device Labeling This action also will make it. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Otc Medical Device Labeling Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). (1) the label of every medical device shall bear a unique device identifier (udi) that meets the requirements of this subpart and part 830 of. In general, labeling for otc medical devices should. This action also will make. Fda Otc Medical Device Labeling.
From emmainternational.com
Discovering FDALabel Your GoTo Labelling Tool Fda Otc Medical Device Labeling This action also will make it. In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. (1) the label of every medical device shall bear a unique. Fda Otc Medical Device Labeling.
From www.vrogue.co
Fda Medical Device Label Symbols vrogue.co Fda Otc Medical Device Labeling Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. This action also. Fda Otc Medical Device Labeling.
From www.lexology.com
FDA Issues Final Rule on Use of Symbols in Labeling Lexology Fda Otc Medical Device Labeling In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. This action also will make it. Labeling regulations pertaining to medical devices are found in the. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT Medical Device Labeling PowerPoint Presentation, free download Fda Otc Medical Device Labeling Fda has finalized its regulation that will require nonprescription drugs to carry clear, simple and readable labeling. In general, labeling for otc medical devices should. The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. (1) the label of every medical device shall bear a unique device identifier (udi) that meets the. Fda Otc Medical Device Labeling.
From www.slideserve.com
PPT FDA LABELING PowerPoint Presentation, free download ID3633953 Fda Otc Medical Device Labeling This action also will make it. Labeling regulations pertaining to medical devices are found in the following parts of title 21 of the code of federal regulations (cfr). The fda’s regulations in 21 cfr part 801, subpart c, set forth specific requirements for labeling otc devices. In general, labeling for otc medical devices should. Fda has finalized its regulation that. Fda Otc Medical Device Labeling.