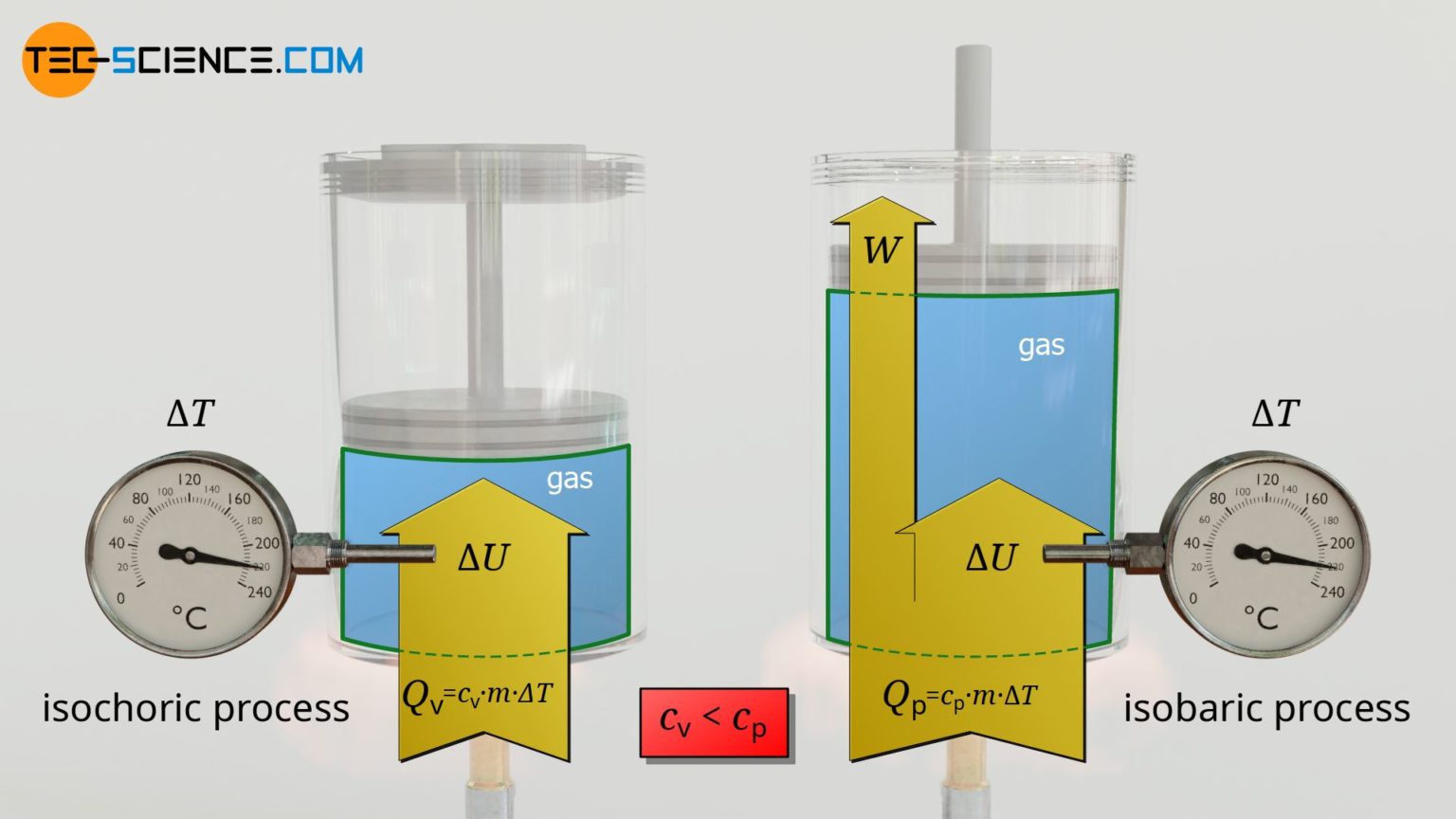
Does Internal Energy Change In An Isobaric Process . In an isobaric process, the pressure does not change, hence \(dp=0\). So the heat added to the gas increases the internal energy and. If the ideal gas was compressed, the heat, the. so if heat energy is added to the system, the temperature increases as does the internal energy. It gives a relationship between the heat. This further means that no quantities, as in the first law of thermodynamics, become zero. isobaric processes can be further understood using the first law of thermodynamics. in the isochoric process, there is no work done. internal energy in isobaric processes. in the process shown in the figure above, the internal energy of the gas increases. However, there is also a change in the internal energy of the system. in an isobaric process, when the heat is transferred to the system, some work is done.
from www.tec-science.com
It gives a relationship between the heat. In an isobaric process, the pressure does not change, hence \(dp=0\). However, there is also a change in the internal energy of the system. If the ideal gas was compressed, the heat, the. internal energy in isobaric processes. So the heat added to the gas increases the internal energy and. in an isobaric process, when the heat is transferred to the system, some work is done. so if heat energy is added to the system, the temperature increases as does the internal energy. isobaric processes can be further understood using the first law of thermodynamics. This further means that no quantities, as in the first law of thermodynamics, become zero.
Isobaric process in a closed system tecscience
Does Internal Energy Change In An Isobaric Process so if heat energy is added to the system, the temperature increases as does the internal energy. However, there is also a change in the internal energy of the system. isobaric processes can be further understood using the first law of thermodynamics. If the ideal gas was compressed, the heat, the. in an isobaric process, when the heat is transferred to the system, some work is done. in the process shown in the figure above, the internal energy of the gas increases. so if heat energy is added to the system, the temperature increases as does the internal energy. So the heat added to the gas increases the internal energy and. It gives a relationship between the heat. in the isochoric process, there is no work done. This further means that no quantities, as in the first law of thermodynamics, become zero. In an isobaric process, the pressure does not change, hence \(dp=0\). internal energy in isobaric processes.
From www.doubtnut.com
[Odia] The change in internal energy in an isobaric process, how repre Does Internal Energy Change In An Isobaric Process So the heat added to the gas increases the internal energy and. so if heat energy is added to the system, the temperature increases as does the internal energy. internal energy in isobaric processes. in an isobaric process, when the heat is transferred to the system, some work is done. If the ideal gas was compressed, the. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Calculating changes in internal energy from equations of state YouTube Does Internal Energy Change In An Isobaric Process However, there is also a change in the internal energy of the system. It gives a relationship between the heat. So the heat added to the gas increases the internal energy and. This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is no work done. If the ideal. Does Internal Energy Change In An Isobaric Process.
From www.mecha-engineeringbd.com
Isobaric Process (Constant Pressure Process) Mechanical Engineering Does Internal Energy Change In An Isobaric Process isobaric processes can be further understood using the first law of thermodynamics. This further means that no quantities, as in the first law of thermodynamics, become zero. So the heat added to the gas increases the internal energy and. However, there is also a change in the internal energy of the system. In an isobaric process, the pressure does. Does Internal Energy Change In An Isobaric Process.
From www.tec-science.com
Internal energy & heat capacity of ideal gases theory of gases Does Internal Energy Change In An Isobaric Process so if heat energy is added to the system, the temperature increases as does the internal energy. in an isobaric process, when the heat is transferred to the system, some work is done. However, there is also a change in the internal energy of the system. In an isobaric process, the pressure does not change, hence \(dp=0\). So. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT “Bioenergetics” Prof. Dr. Metin TULGAR PowerPoint Presentation Does Internal Energy Change In An Isobaric Process in the isochoric process, there is no work done. In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure above, the internal energy of the gas increases. so if heat energy is added to the system, the temperature increases as does the internal energy. However, there is also a. Does Internal Energy Change In An Isobaric Process.
From www.numerade.com
SOLVED in an isobaric process, if work done by the gas is 100 J and Does Internal Energy Change In An Isobaric Process internal energy in isobaric processes. in the process shown in the figure above, the internal energy of the gas increases. This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is no work done. It gives a relationship between the heat. If the ideal gas was compressed,. Does Internal Energy Change In An Isobaric Process.
From www.slideshare.net
Chapter 15=Thermodynamics Does Internal Energy Change In An Isobaric Process In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure above, the internal energy of the gas increases. However, there is also a change in the internal energy of the system. So the heat added to the gas increases the internal energy and. If the ideal gas was compressed, the heat,. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT Isobaric Process PowerPoint Presentation, free download ID4817637 Does Internal Energy Change In An Isobaric Process so if heat energy is added to the system, the temperature increases as does the internal energy. internal energy in isobaric processes. This further means that no quantities, as in the first law of thermodynamics, become zero. isobaric processes can be further understood using the first law of thermodynamics. in an isobaric process, when the heat. Does Internal Energy Change In An Isobaric Process.
From mavink.com
Proceso Isobarico Dibujo Does Internal Energy Change In An Isobaric Process So the heat added to the gas increases the internal energy and. It gives a relationship between the heat. internal energy in isobaric processes. isobaric processes can be further understood using the first law of thermodynamics. This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT THERMODYNAMICS PowerPoint Presentation, free download ID4040289 Does Internal Energy Change In An Isobaric Process In an isobaric process, the pressure does not change, hence \(dp=0\). However, there is also a change in the internal energy of the system. so if heat energy is added to the system, the temperature increases as does the internal energy. It gives a relationship between the heat. in the isochoric process, there is no work done. . Does Internal Energy Change In An Isobaric Process.
From www.grc.nasa.gov
Enthalpy Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. This further means that no quantities, as in the first law of thermodynamics, become zero. isobaric processes can be further understood using the first law of thermodynamics. so if heat energy is added to the system, the temperature increases as does the. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID1549724 Does Internal Energy Change In An Isobaric Process internal energy in isobaric processes. This further means that no quantities, as in the first law of thermodynamics, become zero. If the ideal gas was compressed, the heat, the. in an isobaric process, when the heat is transferred to the system, some work is done. In an isobaric process, the pressure does not change, hence \(dp=0\). It gives. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Thermodynamic Processes Isobaric, Isochoric, Isothermal and Adiabatic Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. So the heat added to the gas increases the internal energy and. in the isochoric process, there is no work done. in an isobaric process, when the heat is transferred to the system, some work is done. isobaric processes can be. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT Adiabatic Processes PowerPoint Presentation, free download ID Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. This further means that no quantities, as in the first law of thermodynamics, become zero. It gives a relationship between the heat. internal energy in isobaric processes. isobaric processes can be further understood using the first law of thermodynamics. If the ideal. Does Internal Energy Change In An Isobaric Process.
From gazemoms.blogspot.com
The Internal Energy Of A System Is Always Increased By gazemoms Does Internal Energy Change In An Isobaric Process internal energy in isobaric processes. So the heat added to the gas increases the internal energy and. In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure above, the internal energy of the gas increases. in an isobaric process, when the heat is transferred to the system, some work. Does Internal Energy Change In An Isobaric Process.
From www.pearson.com
Heat Equations for Isobaric & Isovolumetric Processes Pearson+ Channels Does Internal Energy Change In An Isobaric Process This further means that no quantities, as in the first law of thermodynamics, become zero. in an isobaric process, when the heat is transferred to the system, some work is done. In an isobaric process, the pressure does not change, hence \(dp=0\). so if heat energy is added to the system, the temperature increases as does the internal. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Isobaric Process Thermodynamics Work & Heat Energy, Molar Heat Does Internal Energy Change In An Isobaric Process isobaric processes can be further understood using the first law of thermodynamics. in the process shown in the figure above, the internal energy of the gas increases. If the ideal gas was compressed, the heat, the. so if heat energy is added to the system, the temperature increases as does the internal energy. in an isobaric. Does Internal Energy Change In An Isobaric Process.
From www.chemistrylearner.com
Physical Chemistry Chemistry Learner Does Internal Energy Change In An Isobaric Process in an isobaric process, when the heat is transferred to the system, some work is done. in the process shown in the figure above, the internal energy of the gas increases. If the ideal gas was compressed, the heat, the. So the heat added to the gas increases the internal energy and. This further means that no quantities,. Does Internal Energy Change In An Isobaric Process.
From www.quora.com
What is the change in the internal energy in an isobaric process Does Internal Energy Change In An Isobaric Process However, there is also a change in the internal energy of the system. internal energy in isobaric processes. isobaric processes can be further understood using the first law of thermodynamics. in an isobaric process, when the heat is transferred to the system, some work is done. so if heat energy is added to the system, the. Does Internal Energy Change In An Isobaric Process.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Does Internal Energy Change In An Isobaric Process This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is no work done. in an isobaric process, when the heat is transferred to the system, some work is done. If the ideal gas was compressed, the heat, the. in the process shown in the figure above,. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Change in Entropy of an Isobaric Process YouTube Does Internal Energy Change In An Isobaric Process This further means that no quantities, as in the first law of thermodynamics, become zero. in an isobaric process, when the heat is transferred to the system, some work is done. If the ideal gas was compressed, the heat, the. In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure. Does Internal Energy Change In An Isobaric Process.
From www.tec-science.com
Internal energy & heat capacity of ideal gases theory of gases Does Internal Energy Change In An Isobaric Process in the isochoric process, there is no work done. This further means that no quantities, as in the first law of thermodynamics, become zero. in the process shown in the figure above, the internal energy of the gas increases. However, there is also a change in the internal energy of the system. in an isobaric process, when. Does Internal Energy Change In An Isobaric Process.
From byjus.com
What are the formulae to find the change in internal energy in isobaric Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. It gives a relationship between the heat. internal energy in isobaric processes. in the isochoric process, there is no work done. in an isobaric process, when the heat is transferred to the system, some work is done. isobaric processes can. Does Internal Energy Change In An Isobaric Process.
From courses.lumenlearning.com
Ideal Gas Law Boundless Physics Does Internal Energy Change In An Isobaric Process This further means that no quantities, as in the first law of thermodynamics, become zero. internal energy in isobaric processes. In an isobaric process, the pressure does not change, hence \(dp=0\). It gives a relationship between the heat. in an isobaric process, when the heat is transferred to the system, some work is done. However, there is also. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Derivation of work done in Isobaric Process Kamaldheeriya Maths easy Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. However, there is also a change in the internal energy of the system. in an isobaric process, when the heat is transferred to the system, some work is done. so if heat energy is added to the system, the temperature increases as. Does Internal Energy Change In An Isobaric Process.
From classnotes.org.in
Internal Energy Chemistry, Class 11, Thermodynamics Does Internal Energy Change In An Isobaric Process in the process shown in the figure above, the internal energy of the gas increases. If the ideal gas was compressed, the heat, the. This further means that no quantities, as in the first law of thermodynamics, become zero. So the heat added to the gas increases the internal energy and. isobaric processes can be further understood using. Does Internal Energy Change In An Isobaric Process.
From pressbooks.bccampus.ca
4.1 Internal energy in a system Introduction to Engineering Does Internal Energy Change In An Isobaric Process isobaric processes can be further understood using the first law of thermodynamics. If the ideal gas was compressed, the heat, the. However, there is also a change in the internal energy of the system. So the heat added to the gas increases the internal energy and. in an isobaric process, when the heat is transferred to the system,. Does Internal Energy Change In An Isobaric Process.
From mavink.com
Diagrama Proceso Isobarico Does Internal Energy Change In An Isobaric Process This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is no work done. It gives a relationship between the heat. In an isobaric process, the pressure does not change, hence \(dp=0\). in an isobaric process, when the heat is transferred to the system, some work is done.. Does Internal Energy Change In An Isobaric Process.
From physics.stackexchange.com
thermodynamics In an isothermal process there is no change in Does Internal Energy Change In An Isobaric Process However, there is also a change in the internal energy of the system. This further means that no quantities, as in the first law of thermodynamics, become zero. in the isochoric process, there is no work done. In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure above, the internal. Does Internal Energy Change In An Isobaric Process.
From www.tec-science.com
Isobaric process in a closed system tecscience Does Internal Energy Change In An Isobaric Process so if heat energy is added to the system, the temperature increases as does the internal energy. If the ideal gas was compressed, the heat, the. However, there is also a change in the internal energy of the system. So the heat added to the gas increases the internal energy and. in the isochoric process, there is no. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT Work in Thermodynamic Processes PowerPoint Presentation, free Does Internal Energy Change In An Isobaric Process It gives a relationship between the heat. in the process shown in the figure above, the internal energy of the gas increases. However, there is also a change in the internal energy of the system. In an isobaric process, the pressure does not change, hence \(dp=0\). If the ideal gas was compressed, the heat, the. so if heat. Does Internal Energy Change In An Isobaric Process.
From www.tec-science.com
Isobaric process in a closed system tecscience Does Internal Energy Change In An Isobaric Process in an isobaric process, when the heat is transferred to the system, some work is done. If the ideal gas was compressed, the heat, the. internal energy in isobaric processes. It gives a relationship between the heat. in the isochoric process, there is no work done. isobaric processes can be further understood using the first law. Does Internal Energy Change In An Isobaric Process.
From www.slideserve.com
PPT THERMODYNAMICS PowerPoint Presentation, free download ID6737950 Does Internal Energy Change In An Isobaric Process In an isobaric process, the pressure does not change, hence \(dp=0\). in the process shown in the figure above, the internal energy of the gas increases. So the heat added to the gas increases the internal energy and. internal energy in isobaric processes. so if heat energy is added to the system, the temperature increases as does. Does Internal Energy Change In An Isobaric Process.
From www.youtube.com
Show how internal energy U varies with T in isochoric, isobaric and Does Internal Energy Change In An Isobaric Process If the ideal gas was compressed, the heat, the. isobaric processes can be further understood using the first law of thermodynamics. It gives a relationship between the heat. in the process shown in the figure above, the internal energy of the gas increases. in the isochoric process, there is no work done. This further means that no. Does Internal Energy Change In An Isobaric Process.
From study.com
Determining the Work Done by an Isothermal Process. Chemistry Does Internal Energy Change In An Isobaric Process In an isobaric process, the pressure does not change, hence \(dp=0\). However, there is also a change in the internal energy of the system. If the ideal gas was compressed, the heat, the. internal energy in isobaric processes. It gives a relationship between the heat. This further means that no quantities, as in the first law of thermodynamics, become. Does Internal Energy Change In An Isobaric Process.