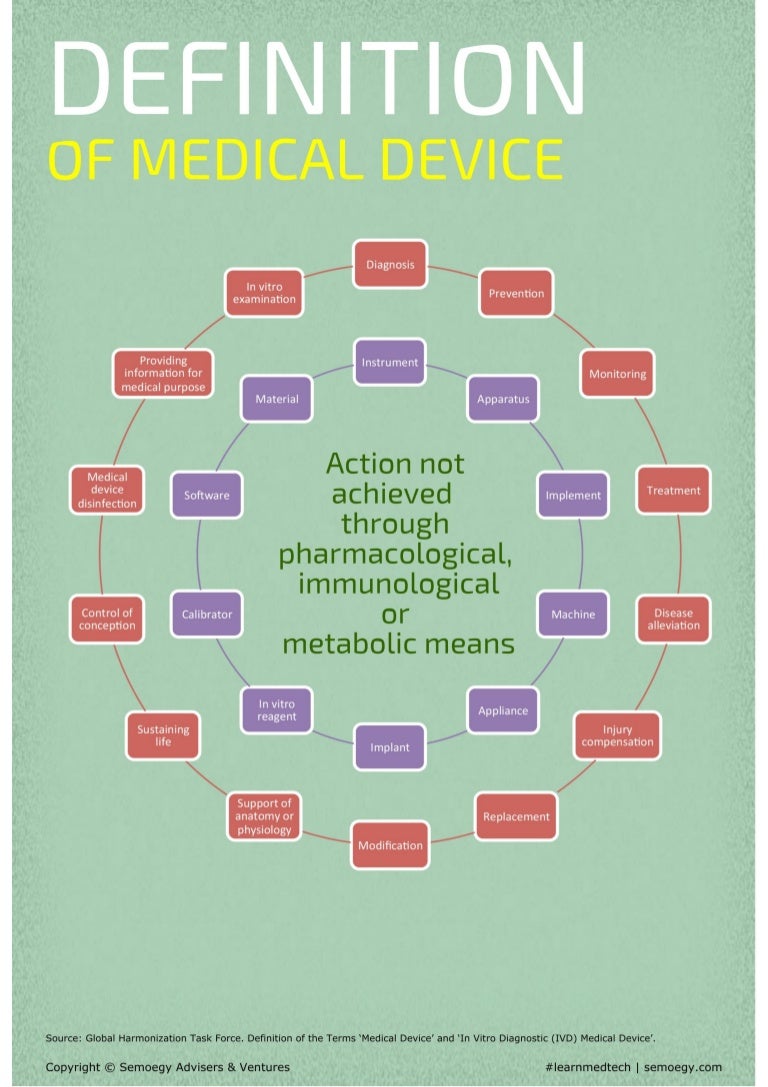
Medical Devices Definition Uk . what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. You need to decide if your product is a medical device before you go through the. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. definition of a medical device. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and.
from de.slideshare.net
definition of a medical device. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. You need to decide if your product is a medical device before you go through the. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage.
Medical device definition
Medical Devices Definition Uk a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. definition of a medical device. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. You need to decide if your product is a medical device before you go through the.
From woodleybioreg.com
Medical devices what are they, and how do we know that they're safe Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a. Medical Devices Definition Uk.
From omcmedical.com
Classification of Medical Devices Based on UK MDR 2002 Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. definition of a medical device. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. what you need to do to place a medical device. Medical Devices Definition Uk.
From www.oxfordcc.co.uk
Software as a Medical Device Oxford Computer Consultants Medical Devices Definition Uk uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage.. Medical Devices Definition Uk.
From www.biosliceblog.com
MHRA’s guide to the new EU Medical Devices Regulations BioSlice Blog Medical Devices Definition Uk the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union. Medical Devices Definition Uk.
From www.slideserve.com
PPT Medical Devices PowerPoint Presentation, free download ID991833 Medical Devices Definition Uk a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. it explains the key steps for placing a medical device on the market in great. Medical Devices Definition Uk.
From www.youtube.com
Medical Device Usability Highlights of European Regulations and the Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. You need to decide if your product is a medical device before you go through. Medical Devices Definition Uk.
From www.slideserve.com
PPT The FDA Landscape AdvaMed September 2008 PowerPoint Presentation Medical Devices Definition Uk uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. definition of a medical device. a medical device is any instrument (other than a medicine) that. Medical Devices Definition Uk.
From mavink.com
Examples Of Medical Devices Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. definition of a medical device. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat. Medical Devices Definition Uk.
From www.arenasolutions.com
How to Classify Your Medical Device for FDA Approval Arena Medical Devices Definition Uk a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. it explains the key steps for placing a medical device on the market in great. Medical Devices Definition Uk.
From cliniexperts.com
ISO 13485 Medical Devices Certification Medical Device ISO Standards Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. definition of a. Medical Devices Definition Uk.
From www.ce-marking.com
Guide on Class I (Is/Im) MDD Medical Devices CE marking (mark Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or. Medical Devices Definition Uk.
From www.sgs.co.uk
Medical Devices SGS UK Medical Devices Definition Uk the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. what you need to do to place a medical device on the great britain, northern ireland. Medical Devices Definition Uk.
From www.qualitymeddev.com
Medical Device Definition according to EU MDR 2017/745 Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. definition. Medical Devices Definition Uk.
From mavink.com
Examples Of Medical Devices Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. definition of a medical device. a medical device is any instrument (other than. Medical Devices Definition Uk.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Devices Definition Uk the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a. Medical Devices Definition Uk.
From talema.com
An Introduction to Medical Electrical Devices The Talema Group Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a. Medical Devices Definition Uk.
From www.slideserve.com
PPT Introduction to Medical Technologies PowerPoint Presentation Medical Devices Definition Uk uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. definition of a medical device. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a medical device is any instrument (other than a medicine) that. Medical Devices Definition Uk.
From www.slideserve.com
PPT Medical Equipment and the Safe Medical Device Act (SMDA Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device. Medical Devices Definition Uk.
From laegemiddelstyrelsen.dk
Medical devices Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. definition of a medical device. the mhra 2023 defines a medical device as any. Medical Devices Definition Uk.
From waddellgrp.com
So you want to make a Medical Device . . . Medical Project Management Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. definition of a medical device. it explains the key steps for placing a medical device on. Medical Devices Definition Uk.
From mungfali.com
Classification Of Medical Devices Medical Devices Definition Uk a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a. Medical Devices Definition Uk.
From www.arenasolutions.com
Medical Device Definition Arena Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. the. Medical Devices Definition Uk.
From medicaldevicehq.com
What is a medical device? Medical Device HQ Medical Devices Definition Uk the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. definition of a medical device. You need to decide if your product is a medical device. Medical Devices Definition Uk.
From laegemiddelstyrelsen.dk
Development of medical devices Medical Devices Definition Uk a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. You need to decide if your product is a medical device before you go through the. definition. Medical Devices Definition Uk.
From spyro-soft.com
EU MDR vs FDA what are the main differences and similarities? Medical Devices Definition Uk a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. a medical device is any instrument (other than a medicine) that is used to. Medical Devices Definition Uk.
From coastbiomed.com
UNDERSTANDING MEDICAL EQUIPMENT CLASSIFICATION Coast Biomedical Equipment Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. uk law specifies requirements that legal manufacturers of medical devices must meet to legally. Medical Devices Definition Uk.
From www.slideserve.com
PPT Life cycle of medical devices PowerPoint Presentation ID254282 Medical Devices Definition Uk uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. it explains the key steps for placing a medical device on the market in great britain (gb). Medical Devices Definition Uk.
From www.researchgate.net
3 Examples of medical devices according to the European and USA Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. You need to decide if your product is a medical device before you go through. Medical Devices Definition Uk.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Definition Uk a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat or manage. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. it explains the key steps for placing a medical device on the market in great britain (gb) and northern. Medical Devices Definition Uk.
From www.youtube.com
What are medical devices? YouTube Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended. Medical Devices Definition Uk.
From de.slideshare.net
Medical device definition Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. definition of a medical device. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. uk law specifies requirements that legal manufacturers of. Medical Devices Definition Uk.
From www.gilero.com
Medical Device Classification Overview of 3 Classes Gilero Medical Devices Definition Uk uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for. Medical Devices Definition Uk.
From angelanjohnson.com
Medical Devices Angela N Johnson Medical Devices Definition Uk You need to decide if your product is a medical device before you go through the. uk law specifies requirements that legal manufacturers of medical devices must meet to legally place a device on the. it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. . Medical Devices Definition Uk.
From reviewmotors.co
Medical Device Directive Spare Parts List Reviewmotors.co Medical Devices Definition Uk it explains the key steps for placing a medical device on the market in great britain (gb) and northern ireland (ni) and. a medical device is any instrument, apparatus, appliance, software, material, or other article, which is intended for human use that performs. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software. Medical Devices Definition Uk.
From www.slideserve.com
PPT Implantable Medical Devices PowerPoint Presentation, free Medical Devices Definition Uk what you need to do to place a medical device on the great britain, northern ireland and european union (eu) markets. the mhra 2023 defines a medical device as any instrument, apparatus, appliance, material software or other article that may. a medical device is any instrument (other than a medicine) that is used to diagnose, monitor, treat. Medical Devices Definition Uk.