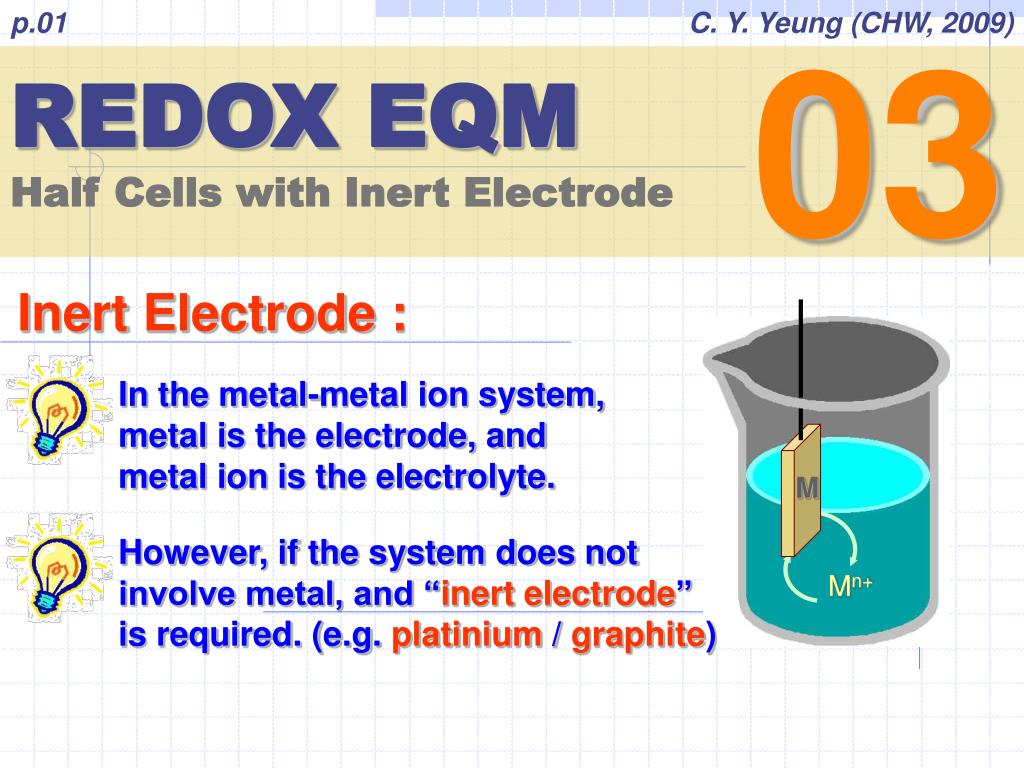
Inert Electrode Chemistry Examples . Platinum or gold generally make good inert electrodes because they are chemically unreactive. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Some commonly used inert electrodes: Graphite(carbon), platinum, gold, and rhodium. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Cucl2 → cu + cl2. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Copper ions move to the cathode where solid copper (cu) is. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a.
from www.slideserve.com
Some commonly used inert electrodes: Platinum or gold generally make good inert electrodes because they are chemically unreactive. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Copper ions move to the cathode where solid copper (cu) is. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Cucl2 → cu + cl2. Graphite(carbon), platinum, gold, and rhodium.
PPT REDOX EQM Half Cells with Inert Electrode PowerPoint Presentation
Inert Electrode Chemistry Examples the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Copper ions move to the cathode where solid copper (cu) is. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Cucl2 → cu + cl2. Graphite(carbon), platinum, gold, and rhodium. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Some commonly used inert electrodes: the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Platinum or gold generally make good inert electrodes because they are chemically unreactive.
From www.youtube.com
Inert electrodes Trew’s notes YouTube Inert Electrode Chemistry Examples Some commonly used inert electrodes: An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail. Inert Electrode Chemistry Examples.
From byjus.com
What is an active electrode? Explain with the help of an example Inert Electrode Chemistry Examples An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Graphite(carbon), platinum, gold, and rhodium. the main difference between active and inert electrodes is that active electrodes participate. Inert Electrode Chemistry Examples.
From library.fiveable.me
Electrolysis Intro to Chemistry Class Notes Fiveable Inert Electrode Chemistry Examples Platinum or gold generally make good inert electrodes because they are chemically unreactive. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail at the electrolysis of solutions of ionic compounds using inert. Inert Electrode Chemistry Examples.
From www.youtube.com
Ch31 Redox Reactions in Chemical Cells (Inert electrode, Easy examples Inert Electrode Chemistry Examples Cucl2 → cu + cl2. Copper ions move to the cathode where solid copper (cu) is. Graphite(carbon), platinum, gold, and rhodium. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. the main difference between active and inert electrodes is that active electrodes participate in the. Inert Electrode Chemistry Examples.
From www.youtube.com
'O' Level Chemistry Molten Electrolyte + Inert Electrode YouTube Inert Electrode Chemistry Examples This is an electrode that is made of a substance that does not undergo oxidation or reduction. Graphite(carbon), platinum, gold, and rhodium. Cucl2 → cu + cl2. Some commonly used inert electrodes: the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo. Inert Electrode Chemistry Examples.
From www.chegg.com
Solved Select all examples of an inert electrode. Check all Inert Electrode Chemistry Examples the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Platinum or gold generally make good inert electrodes because they are. Inert Electrode Chemistry Examples.
From studygreywether.z13.web.core.windows.net
What Is Inert Electrode Inert Electrode Chemistry Examples This is an electrode that is made of a substance that does not undergo oxidation or reduction. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Platinum or gold generally make good inert electrodes because they are chemically unreactive. Graphite(carbon), platinum, gold, and rhodium. the main difference between active and. Inert Electrode Chemistry Examples.
From diagramwallsgymnasial.z13.web.core.windows.net
Simple Electric Cell Diagram Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. Copper ions move to the cathode where solid copper (cu) is. Cucl2 → cu + cl2. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system,. Inert Electrode Chemistry Examples.
From studylibplimsoll.z21.web.core.windows.net
What Is An Inert Electrode Inert Electrode Chemistry Examples Cucl2 → cu + cl2. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Platinum. Inert Electrode Chemistry Examples.
From www.youtube.com
Electrolysis of dilute sodium chloride (inert electrodes) YouTube Inert Electrode Chemistry Examples Copper ions move to the cathode where solid copper (cu) is. Cucl2 → cu + cl2. Some commonly used inert electrodes: This is an electrode that is made of a substance that does not undergo oxidation or reduction. Platinum or gold generally make good inert electrodes because they are chemically unreactive. this page looks in detail at the electrolysis. Inert Electrode Chemistry Examples.
From brainly.in
There are two types of electrodes and they are Active electrode Inert Inert Electrode Chemistry Examples Some commonly used inert electrodes: An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Graphite(carbon), platinum, gold, and rhodium. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. This. Inert Electrode Chemistry Examples.
From studylibrenegado.z4.web.core.windows.net
Inert Electrodes Bbc Bitesize Inert Electrode Chemistry Examples Some commonly used inert electrodes: Platinum or gold generally make good inert electrodes because they are chemically unreactive. Copper ions move to the cathode where solid copper (cu) is. Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Cucl2 → cu + cl2. This is an. Inert Electrode Chemistry Examples.
From studycopesettic.z21.web.core.windows.net
Inert Electrodes Gcse Inert Electrode Chemistry Examples the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Some commonly used inert electrodes: Copper ions move to the cathode where solid copper (cu) is. this page looks in detail at the electrolysis of solutions of ionic. Inert Electrode Chemistry Examples.
From saylordotorg.github.io
Electrochemistry Inert Electrode Chemistry Examples An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Graphite(carbon), platinum, gold, and rhodium. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail. Inert Electrode Chemistry Examples.
From www.nagwa.com
Question Video Writing the Equation for the Reaction at the Anode Inert Electrode Chemistry Examples An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail at the electrolysis of solutions. Inert Electrode Chemistry Examples.
From www.youtube.com
Electrolysis of dilute sulfuric acid (inert electrodes) YouTube Inert Electrode Chemistry Examples Platinum or gold generally make good inert electrodes because they are chemically unreactive. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical. Inert Electrode Chemistry Examples.
From www.slideshare.net
Electrolysis part 3 aqueous solution Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. Some commonly used inert electrodes: this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. This is an electrode that is made of a. Inert Electrode Chemistry Examples.
From www.youtube.com
4 Inert Electrodes YouTube Inert Electrode Chemistry Examples Some commonly used inert electrodes: Graphite(carbon), platinum, gold, and rhodium. Cucl2 → cu + cl2. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Platinum or gold generally make good inert electrodes because they are chemically unreactive. This is an electrode that is made of a substance that does not undergo. Inert Electrode Chemistry Examples.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Copper ions move to the cathode where solid copper (cu) is. This is an electrode that is made of a substance that does not undergo oxidation or reduction. the main difference. Inert Electrode Chemistry Examples.
From fyorocyxj.blob.core.windows.net
Electrode Definition Chemistry Gcse at Kathryn Patton blog Inert Electrode Chemistry Examples this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Cucl2 → cu + cl2. Some commonly used inert electrodes: Platinum or gold generally make good inert electrodes because they are chemically unreactive. the main difference between active and inert electrodes is that active electrodes participate. Inert Electrode Chemistry Examples.
From www.slideserve.com
PPT REDOX EQM Half Cells with Inert Electrode PowerPoint Presentation Inert Electrode Chemistry Examples This is an electrode that is made of a substance that does not undergo oxidation or reduction. Platinum or gold generally make good inert electrodes because they are chemically unreactive. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical. Inert Electrode Chemistry Examples.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube Inert Electrode Chemistry Examples Cucl2 → cu + cl2. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. An. Inert Electrode Chemistry Examples.
From www.youtube.com
Ch.5 Electrolysis of aq. copper sulfate using carbon electrode (inert Inert Electrode Chemistry Examples Cucl2 → cu + cl2. Some commonly used inert electrodes: Copper ions move to the cathode where solid copper (cu) is. This is an electrode that is made of a substance that does not undergo oxidation or reduction. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or. Inert Electrode Chemistry Examples.
From mungfali.com
Types Of Electrodes Inert Electrode Chemistry Examples Some commonly used inert electrodes: Copper ions move to the cathode where solid copper (cu) is. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. This is an electrode that is made of a substance that does not undergo oxidation or reduction. An inert electrode is. Inert Electrode Chemistry Examples.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Copper ions move to the cathode where solid copper (cu) is. Platinum or gold generally make good inert electrodes because they are chemically unreactive.. Inert Electrode Chemistry Examples.
From exypbyonv.blob.core.windows.net
What Are Inert Electrodes In Electrolysis at Gene Dickson blog Inert Electrode Chemistry Examples this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. Copper ions move to the cathode where solid copper (cu) is. Platinum or gold generally make good inert electrodes because they are chemically unreactive. Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductor that does. Inert Electrode Chemistry Examples.
From www.youtube.com
Chemistry Electrolysis (Inert & Active Electrodes) YouTube Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Platinum or gold generally make good inert electrodes because they are chemically unreactive. this page looks in detail at. Inert Electrode Chemistry Examples.
From www.slideserve.com
PPT Lets Set The Stage PowerPoint Presentation, free download ID Inert Electrode Chemistry Examples Some commonly used inert electrodes: Copper ions move to the cathode where solid copper (cu) is. Cucl2 → cu + cl2. Platinum or gold generally make good inert electrodes because they are chemically unreactive. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do. Inert Electrode Chemistry Examples.
From www.differencebetween.com
Difference Between Active and Inert Electrodes Compare the Difference Inert Electrode Chemistry Examples This is an electrode that is made of a substance that does not undergo oxidation or reduction. Graphite(carbon), platinum, gold, and rhodium. Some commonly used inert electrodes: this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon (graphite) or platinum. An inert electrode is a conductor that does not participate. Inert Electrode Chemistry Examples.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. Copper ions move to the cathode where solid copper (cu) is. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. this page looks in detail at the electrolysis of solutions of ionic. Inert Electrode Chemistry Examples.
From saylordotorg.github.io
Describing Electrochemical Cells Inert Electrode Chemistry Examples Some commonly used inert electrodes: Platinum or gold generally make good inert electrodes because they are chemically unreactive. Cucl2 → cu + cl2. Copper ions move to the cathode where solid copper (cu) is. Graphite(carbon), platinum, gold, and rhodium. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a. Inert Electrode Chemistry Examples.
From chem.libretexts.org
6.2 Standard Electrode Potentials Chemistry LibreTexts Inert Electrode Chemistry Examples This is an electrode that is made of a substance that does not undergo oxidation or reduction. Platinum or gold generally make good inert electrodes because they are chemically unreactive. Some commonly used inert electrodes: Graphite(carbon), platinum, gold, and rhodium. this page looks in detail at the electrolysis of solutions of ionic compounds using inert electrodes such as carbon. Inert Electrode Chemistry Examples.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. This is an electrode that is made of a substance that does not undergo oxidation or reduction. Some commonly used inert electrodes: the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Platinum or. Inert Electrode Chemistry Examples.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes Inert Electrode Chemistry Examples Copper ions move to the cathode where solid copper (cu) is. the main difference between active and inert electrodes is that active electrodes participate in the electrochemical reactions occurring in a system, while inert electrodes do not undergo any chemical changes. Platinum or gold generally make good inert electrodes because they are chemically unreactive. An inert electrode is a. Inert Electrode Chemistry Examples.
From www.youtube.com
Ch31 part 1 chemical cell with inert electrodes YouTube Inert Electrode Chemistry Examples Graphite(carbon), platinum, gold, and rhodium. An inert electrode is a conductor that does not participate in the electrochemical reaction but serves as a. Platinum or gold generally make good inert electrodes because they are chemically unreactive. Cucl2 → cu + cl2. Copper ions move to the cathode where solid copper (cu) is. Some commonly used inert electrodes: the main. Inert Electrode Chemistry Examples.