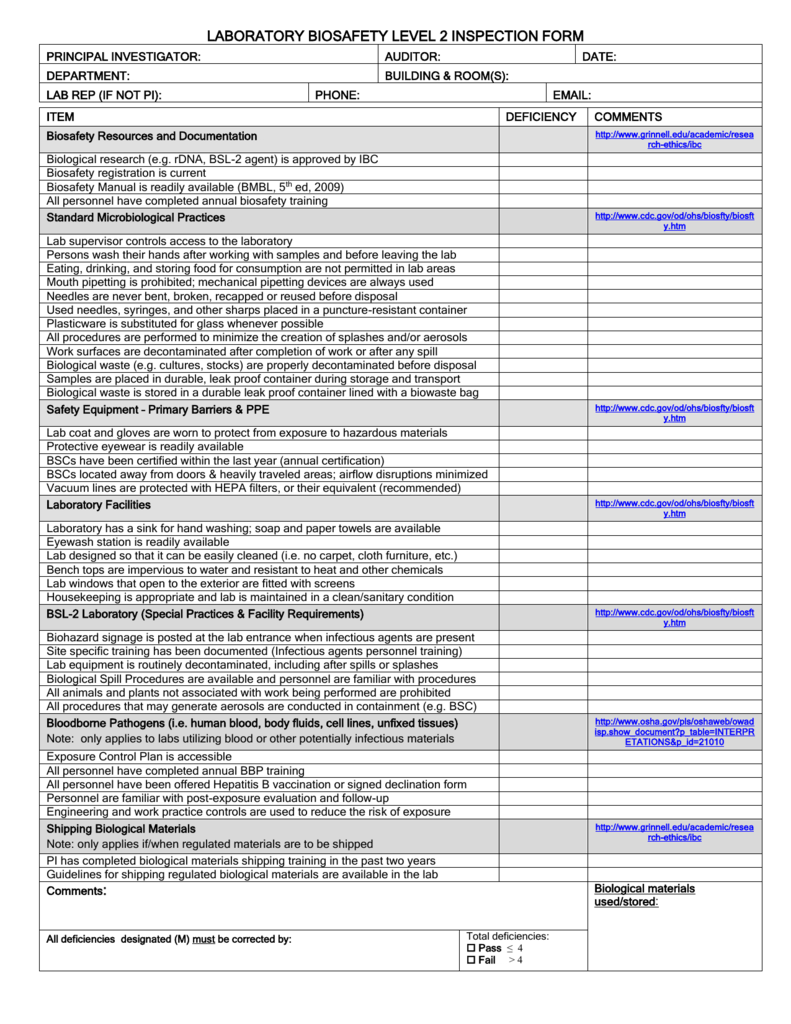
Cap Laboratory Personnel Requirements . Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Maintain accuracy of test results and ensure accurate patient diagnosis. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Delve into insights from a seasoned cap. Cap requirements commonly exceed the. (a) possess a current license issued by the state in which the laboratory is located, if. Each individual performing high complexity testing must—.
from exoumxnen.blob.core.windows.net
Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Delve into insights from a seasoned cap. Each individual performing high complexity testing must—. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requirements commonly exceed the. Meet required standards from clia, fda and osha. (a) possess a current license issued by the state in which the laboratory is located, if. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Maintain accuracy of test results and ensure accurate patient diagnosis. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or.
Cap Lab Regulations at David Quintanilla blog
Cap Laboratory Personnel Requirements Meet required standards from clia, fda and osha. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Delve into insights from a seasoned cap. Maintain accuracy of test results and ensure accurate patient diagnosis. Each individual performing high complexity testing must—. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requirements commonly exceed the. (a) possess a current license issued by the state in which the laboratory is located, if.
From exoumxnen.blob.core.windows.net
Cap Lab Regulations at David Quintanilla blog Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requirements commonly exceed the. Meet required standards from clia, fda and osha. No. Cap Laboratory Personnel Requirements.
From www.mlo-online.com
CLIA and regulatory readiness How can your lab always be ready? Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Delve into insights from a seasoned cap. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. (a) possess a current license issued by. Cap Laboratory Personnel Requirements.
From www.pdffiller.com
CAP Laboratory Accreditation Program Protocol Required Use Date March Cap Laboratory Personnel Requirements Maintain accuracy of test results and ensure accurate patient diagnosis. Meet required standards from clia, fda and osha. Each individual performing high complexity testing must—. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. (a) possess a current license issued by the state in which the. Cap Laboratory Personnel Requirements.
From www.scribd.com
CAP Accreditation Requirements For Validation of Laboratory Tests PDF Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. (a). Cap Laboratory Personnel Requirements.
From www.mlo-online.com
CLIA and regulatory readiness How can your lab always be ready? Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Each individual performing high complexity testing must—. Delve into insights from a seasoned cap. Cap requirements commonly exceed the. Maintain accuracy. Cap Laboratory Personnel Requirements.
From www.complianceonline.com
cGMP and GLP Regulations for Quality Control Labs An overview Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Each individual performing high complexity testing must—. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Delve into insights from a seasoned cap. Maintain accuracy of test results and ensure. Cap Laboratory Personnel Requirements.
From www.lighthouselabservices.com
What Is a CLIA Lab Director and What Are Their Requirements? Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Maintain accuracy of test results and ensure accurate patient diagnosis. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Delve into insights from a seasoned cap. Cap requires that all high complexity testing personnel have earned at. Cap Laboratory Personnel Requirements.
From www.scribd.com
CLIA Requirements For Proficiency Testing The Basics For Laboratory Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Maintain accuracy of test results and ensure accurate patient diagnosis. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Meet required standards from clia, fda and osha. Delve into insights from a seasoned. Cap Laboratory Personnel Requirements.
From www.youtube.com
CAP Accreditation 10 Steps on the Path to Laboratory Excellence YouTube Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Delve into insights from a seasoned cap. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requirements commonly exceed the. Maintain accuracy of test results and ensure accurate patient. Cap Laboratory Personnel Requirements.
From www.slideserve.com
PPT for Molecular Proficiency Testing Cap Laboratory Personnel Requirements Meet required standards from clia, fda and osha. (a) possess a current license issued by the state in which the laboratory is located, if. Maintain accuracy of test results and ensure accurate patient diagnosis. Each individual performing high complexity testing must—. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires that. Cap Laboratory Personnel Requirements.
From mungfali.com
Lab Safety Rules Poster Cap Laboratory Personnel Requirements Delve into insights from a seasoned cap. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. (a) possess a current license issued by the state in. Cap Laboratory Personnel Requirements.
From www.slideserve.com
PPT What Every USAF Laboratorian Should Know PowerPoint Presentation Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Maintain accuracy of test results. Cap Laboratory Personnel Requirements.
From cerbaresearch.com
Northwell Health Laboratories CAP certification Cerba Research Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory. Cap Laboratory Personnel Requirements.
From www.slideserve.com
PPT What Every USAF Laboratorian Should Know PowerPoint Presentation Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Each individual performing high complexity testing must—. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires that all high complexity testing personnel have earned at least a minimum. Cap Laboratory Personnel Requirements.
From www.slideserve.com
PPT What Every USAF Laboratorian Should Know PowerPoint Presentation Cap Laboratory Personnel Requirements (a) possess a current license issued by the state in which the laboratory is located, if. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Delve into insights from a seasoned cap. Cap requirements commonly exceed the. Cap requires that all high complexity testing personnel have. Cap Laboratory Personnel Requirements.
From mungfali.com
Sample Competency Assessment Forms Cap Laboratory Personnel Requirements Maintain accuracy of test results and ensure accurate patient diagnosis. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. Delve into insights from a seasoned cap. Cap requires that all high complexity testing personnel have earned at least. Cap Laboratory Personnel Requirements.
From www.templateroller.com
Form CMS209 Fill Out, Sign Online and Download Printable PDF Cap Laboratory Personnel Requirements Maintain accuracy of test results and ensure accurate patient diagnosis. Delve into insights from a seasoned cap. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requirements commonly exceed the. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires. Cap Laboratory Personnel Requirements.
From www.pdffiller.com
Fillable Online CAP lab accreditation/checklists/protocols/guidelines Cap Laboratory Personnel Requirements Cap requirements commonly exceed the. Each individual performing high complexity testing must—. (a) possess a current license issued by the state in which the laboratory is located, if. Maintain accuracy of test results and ensure accurate patient diagnosis. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requires. Cap Laboratory Personnel Requirements.
From www.dreamstime.com
Lab Safety Protocols Poster Stock Vector Illustration of information Cap Laboratory Personnel Requirements Meet required standards from clia, fda and osha. Each individual performing high complexity testing must—. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Delve into insights from a seasoned cap. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requires. Cap Laboratory Personnel Requirements.
From www.slideserve.com
PPT What Every USAF Laboratorian Should Know PowerPoint Presentation Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Delve into insights from a seasoned cap. Meet required standards from clia, fda and osha. Each individual. Cap Laboratory Personnel Requirements.
From hxegmqlxf.blob.core.windows.net
Cap Requirements For Lab Personnel at Angela Randall blog Cap Laboratory Personnel Requirements Each individual performing high complexity testing must—. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requirements commonly exceed the. Meet required standards from clia,. Cap Laboratory Personnel Requirements.
From www.vrogue.co
New Lab Coat And Ppe Posters Environmental Health Saf vrogue.co Cap Laboratory Personnel Requirements Cap requirements commonly exceed the. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived. Cap Laboratory Personnel Requirements.
From www.vecteezy.com
Laboratory personal protective equipment infographic 1229477 Vector Art Cap Laboratory Personnel Requirements Each individual performing high complexity testing must—. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Meet required standards from clia, fda and osha. Cap requirements commonly exceed the. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a. Cap Laboratory Personnel Requirements.
From americanboardofoptometry.org
CAP Requirements American Board of Optometry Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. Maintain accuracy of test results and ensure accurate patient diagnosis. No specific requirements. Cap Laboratory Personnel Requirements.
From www.studocu.com
Laboratory Safety AND Regulations LABORATORY SAFETY AND REGULATIONS Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Delve. Cap Laboratory Personnel Requirements.
From www.chemistry.ucla.edu
Personal Protective Equipment UCLA Chemistry and Biochemistry Cap Laboratory Personnel Requirements Cap requirements commonly exceed the. (a) possess a current license issued by the state in which the laboratory is located, if. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requires that all high complexity testing personnel have earned at least a minimum of an. Cap Laboratory Personnel Requirements.
From newsroom.cap.org
CAP Secures CMS Reapproval as Accreditation Organization Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Each individual performing high complexity testing must—. Meet required standards from clia, fda and osha. (a) possess a current license issued by the state in which the laboratory is located, if. Delve into insights from a seasoned. Cap Laboratory Personnel Requirements.
From www.kewaunee.in
Designing a Laboratory Safety and Regulatory Requirements Kewaunee Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. Cap requires that all high complexity testing personnel have earned at least a. Cap Laboratory Personnel Requirements.
From hxegmqlxf.blob.core.windows.net
Cap Requirements For Lab Personnel at Angela Randall blog Cap Laboratory Personnel Requirements Maintain accuracy of test results and ensure accurate patient diagnosis. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Meet required standards from clia, fda and osha. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. (a) possess a. Cap Laboratory Personnel Requirements.
From studylib.net
Medical laboratory Personnel Act 2013 Cap Laboratory Personnel Requirements Each individual performing high complexity testing must—. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requirements commonly exceed the. Meet required standards from clia, fda and osha. Maintain accuracy of test. Cap Laboratory Personnel Requirements.
From exotijeyh.blob.core.windows.net
Cap Requirements For Competency Assessment at Sharon Mart blog Cap Laboratory Personnel Requirements Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. Maintain accuracy of test results and. Cap Laboratory Personnel Requirements.
From vdocuments.mx
CLIA Clinical Laboratory Personnel Requirements … Clinical Laboratory Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requirements commonly exceed the. (a) possess a current license issued by the state in which the laboratory is located, if. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Each individual performing. Cap Laboratory Personnel Requirements.
From www.uslegalforms.com
Competency Assessment Clinical Laboratory Personnel Form 20202021 Cap Laboratory Personnel Requirements Explore the latest updates in laboratory personnel qualifications and competency requirements under the new cms regulations. Cap requirements commonly exceed the. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Each individual performing high complexity testing must—. Cap requires that all high complexity testing personnel have. Cap Laboratory Personnel Requirements.
From www.g2intelligence.com
An Analysis of CMS’s Proposed Changes to CLIA Lab Personnel Requirements Cap Laboratory Personnel Requirements Delve into insights from a seasoned cap. Meet required standards from clia, fda and osha. Each individual performing high complexity testing must—. (a) possess a current license issued by the state in which the laboratory is located, if. No specific requirements outlined in the cap or clia regulations, however each laboratory must ensure waived testing personnel meet facility. Explore the. Cap Laboratory Personnel Requirements.
From www.pdffiller.com
laboratory competency assessment checklist Doc Template pdfFiller Cap Laboratory Personnel Requirements Cap requirements commonly exceed the. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Cap requires that all high complexity testing personnel have earned at least a minimum of an associate degree in a laboratory science or. Meet required standards from clia, fda and osha. No. Cap Laboratory Personnel Requirements.