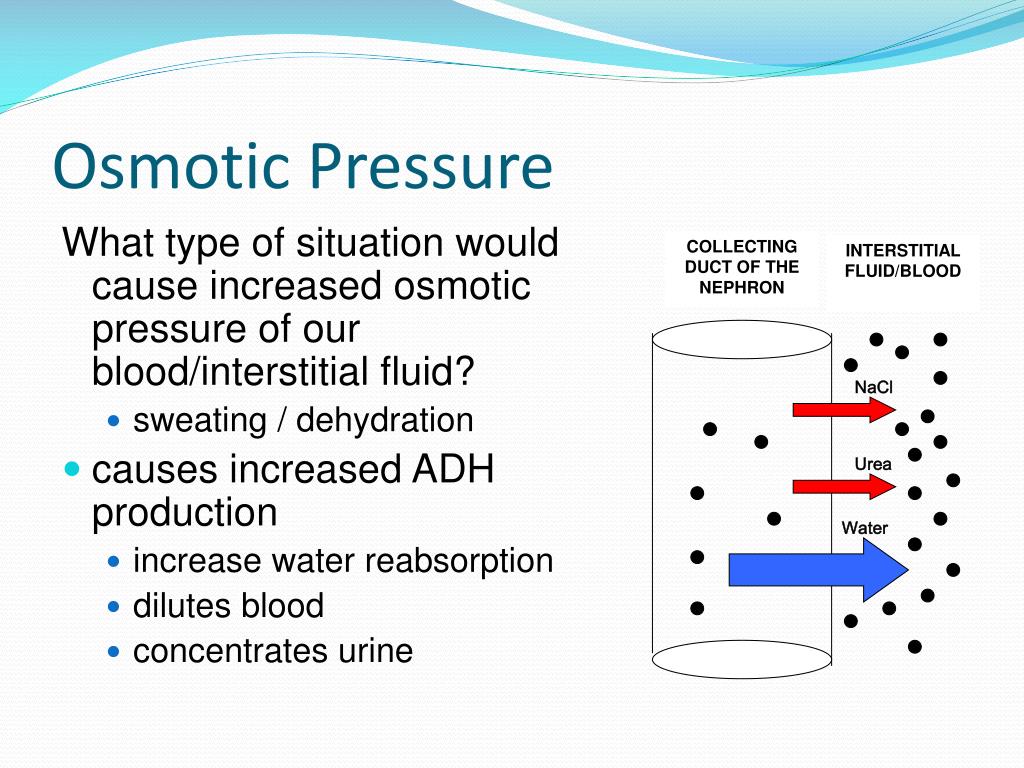
Osmotic Pressure Of Normal Saline . It is a crystalloid fluid administered via an intravenous solution. Osmolarity is defined as the. It has an osmolality of 308. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting.
from www.slideserve.com
The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It is a crystalloid fluid administered via an intravenous solution. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). It has an osmolality of 308. Osmolarity is defined as the.
PPT Water Balance PowerPoint Presentation, free download ID4769466
Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. It has an osmolality of 308. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Osmolarity is defined as the. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. It is a crystalloid fluid administered via an intravenous solution. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to.
From www.researchgate.net
Changes in osmotic pressure of optimized nanoemulsion formulation F4 Osmotic Pressure Of Normal Saline It has an osmolality of 308. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Assuming an ideal solution, osmotic pressure (π) in mmhg is. Osmotic Pressure Of Normal Saline.
From chem.libretexts.org
3.6 Osmotic Pressure Chemistry LibreTexts Osmotic Pressure Of Normal Saline It is a crystalloid fluid administered via an intravenous solution. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline solution (0.9%. Osmotic Pressure Of Normal Saline.
From www.studypool.com
SOLUTION Physical chemistry osmotic pressure and its measurement Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. It is a crystalloid fluid administered via an intravenous solution. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. The osmotic pressure is. Osmotic Pressure Of Normal Saline.
From www.youtube.com
osmosis and osmotic pressure solutions solutions class 12 chemistry Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It is a crystalloid fluid administered via an intravenous solution. Normal saline solution (0.9%. Osmotic Pressure Of Normal Saline.
From www.chemistryland.com
Colligative Properties Osmotic Pressure Of Normal Saline Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. It has an osmolality of 308. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable. Osmotic Pressure Of Normal Saline.
From www.numerade.com
SOLVED A 0.86 by mass solution of NaCl is called "physiological Osmotic Pressure Of Normal Saline Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It is a crystalloid fluid administered via an intravenous solution. The osmotic pressure is the hydrostatic (or hydraulic) pressure required. Osmotic Pressure Of Normal Saline.
From www.researchgate.net
2 The osmotic pressure of possible five sources of concentrated saline Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. It has an osmolality of 308. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small. Osmotic Pressure Of Normal Saline.
From www.slideserve.com
PPT Osmosis, Osmotic pressure and Molecular processes PowerPoint Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that. Osmotic Pressure Of Normal Saline.
From www.priyamstudycentre.com
Osmosis Definition, Example, Osmotic Pressure Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. It has an osmolality of 308. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline. Osmotic Pressure Of Normal Saline.
From doctorlib.info
The Cell Membrane Physiology An Illustrated Review Osmotic Pressure Of Normal Saline It has an osmolality of 308. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. It is a crystalloid fluid administered via an intravenous solution. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Osmolarity is defined as the. Normal saline. Osmotic Pressure Of Normal Saline.
From pharmaceuticalmicrobiologi.blogspot.com
PHARMACEUTICAL MICROBIOLOGY Osmotic Pressure Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Osmolarity is defined as the. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. It is a crystalloid fluid administered via an intravenous solution. Assuming. Osmotic Pressure Of Normal Saline.
From www.slideserve.com
PPT Intravenous Therapy IV Infusion Preparations Fluid and Osmotic Pressure Of Normal Saline Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). The osmotic pressure is the hydrostatic (or hydraulic) pressure required to. Osmotic Pressure Of Normal Saline.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It has an osmolality of 308. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Normal saline contains 0.91% w/v. Osmotic Pressure Of Normal Saline.
From www.numerade.com
SOLVED Normal saline is a solution of aqueous sodium chloride with a Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement. Osmotic Pressure Of Normal Saline.
From slideplayer.com
Westmead Hospital Primary teaching series ppt download Osmotic Pressure Of Normal Saline The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154. Osmotic Pressure Of Normal Saline.
From courses.lumenlearning.com
Kinds of Transport Biology for Majors I Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It has an osmolality of 308. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Osmolarity is defined as the. Normal saline solution (0.9% nacl) or nss, is a. Osmotic Pressure Of Normal Saline.
From www.researchgate.net
Effect of the osmotic pressure of salt solutions (NaHCO 3 , NaCl and Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It is a crystalloid fluid administered via an intravenous solution. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Osmolarity is defined as. Osmotic Pressure Of Normal Saline.
From www.biologyonline.com
Osmotic pressure Definition and Examples Biology Online Dictionary Osmotic Pressure Of Normal Saline It is a crystalloid fluid administered via an intravenous solution. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. The osmotic pressure is the hydrostatic (or hydraulic) pressure required. Osmotic Pressure Of Normal Saline.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Of Normal Saline Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that. Osmotic Pressure Of Normal Saline.
From www.numerade.com
SOLVED Use the isotonic saline solution, 0.92 NaCl (mass/volume), to Osmotic Pressure Of Normal Saline Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. It has an osmolality of 308. The osmotic pressure is the. Osmotic Pressure Of Normal Saline.
From www.derangedphysiology.com
Response to 1L of normal saline Deranged Physiology Osmotic Pressure Of Normal Saline Osmolarity is defined as the. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. It has an osmolality of 308. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium. Osmotic Pressure Of Normal Saline.
From www.youtube.com
Osmotic Pressure Osmosis Colligative property Physiology Series Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to. Osmotic Pressure Of Normal Saline.
From inspiritvr.com
Osmotic Pressure Study Guide Inspirit Osmotic Pressure Of Normal Saline Osmolarity is defined as the. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. It is a crystalloid fluid administered via an intravenous solution. Assuming an ideal solution, osmotic. Osmotic Pressure Of Normal Saline.
From thechemistrynotes.com
Osmotic Pressure Definition, Formulas, Laws Osmotic Pressure Of Normal Saline The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. It has an osmolality of 308. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water,. Osmotic Pressure Of Normal Saline.
From www.researchgate.net
Osmotic pressure for different solutions [1]. In conclusion, in order Osmotic Pressure Of Normal Saline Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. The osmotic pressure is the hydrostatic (or hydraulic). Osmotic Pressure Of Normal Saline.
From kunduz.com
What Is Osmosis? Definition, Types, Significance, Osmotic Pressure Kunduz Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. It is a crystalloid fluid administered via an intravenous solution. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Assuming an ideal solution,. Osmotic Pressure Of Normal Saline.
From www.numerade.com
A 0.86 percent by mass solution of NaCl is called "physiological saline Osmotic Pressure Of Normal Saline Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Because normal saline is isoosmotic with the extracellular fluid, water does not have any osmotic pressure to shift between compartments, and it distributes itself according to. Assuming an ideal solution, osmotic pressure (π) in. Osmotic Pressure Of Normal Saline.
From general.chemistrysteps.com
Osmotic Pressure Chemistry Steps Osmotic Pressure Of Normal Saline Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. It has an osmolality of 308. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Assuming an. Osmotic Pressure Of Normal Saline.
From wizedu.com
Blood generates an osmotic pressure equivalent to the pressure Osmotic Pressure Of Normal Saline Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Osmolarity is defined as the. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times. Osmotic Pressure Of Normal Saline.
From readchemistry.com
Laws of Osmotic Pressure Read Chemistry Osmotic Pressure Of Normal Saline It has an osmolality of 308. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Osmolarity is defined as the. It is a crystalloid fluid administered via an intravenous solution. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical. Osmotic Pressure Of Normal Saline.
From pcrf.princeton.edu
MIPSE invited talk “Osmotic Pressure on Cell Membranes in a Saline Osmotic Pressure Of Normal Saline Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Normal saline. Osmotic Pressure Of Normal Saline.
From www.chemistrylearner.com
Osmotic Pressure Definition, Formula, and Examples Osmotic Pressure Of Normal Saline Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow solvent molecules to. The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Normal saline. Osmotic Pressure Of Normal Saline.
From questions-in.kunduz.com
What is the osmotic pressure of an isotoni... Organic Chemistry Osmotic Pressure Of Normal Saline The osmotic pressure is the hydrostatic (or hydraulic) pressure required to oppose the movement of water through a. Osmolarity is defined as the. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Osmotic pressure is a colligative property of solutions that is observed using a semipermeable membrane, a barrier with pores small enough to allow. Osmotic Pressure Of Normal Saline.
From www.slideserve.com
PPT Intravenous Therapy PowerPoint Presentation, free download ID Osmotic Pressure Of Normal Saline It is a crystalloid fluid administered via an intravenous solution. Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. Normal saline solution (0.9% nacl) or nss, is a crystalloid isotonic iv fluid that contains water, sodium (154 meq/l), and chloride (154 meq/l). Assuming an ideal solution, osmotic pressure (π). Osmotic Pressure Of Normal Saline.
From www.slideserve.com
PPT Water Balance PowerPoint Presentation, free download ID4769466 Osmotic Pressure Of Normal Saline Normal saline contains 0.91% w/v of sodium chloride, corresponding to 0.154 m, making its osmotic pressure close to that of. It has an osmolality of 308. Assuming an ideal solution, osmotic pressure (π) in mmhg is 19.3 times the osmolarity. Normal saline is a cornerstone of intravenous solutions commonly used in the clinical setting. Osmotic pressure is a colligative property. Osmotic Pressure Of Normal Saline.