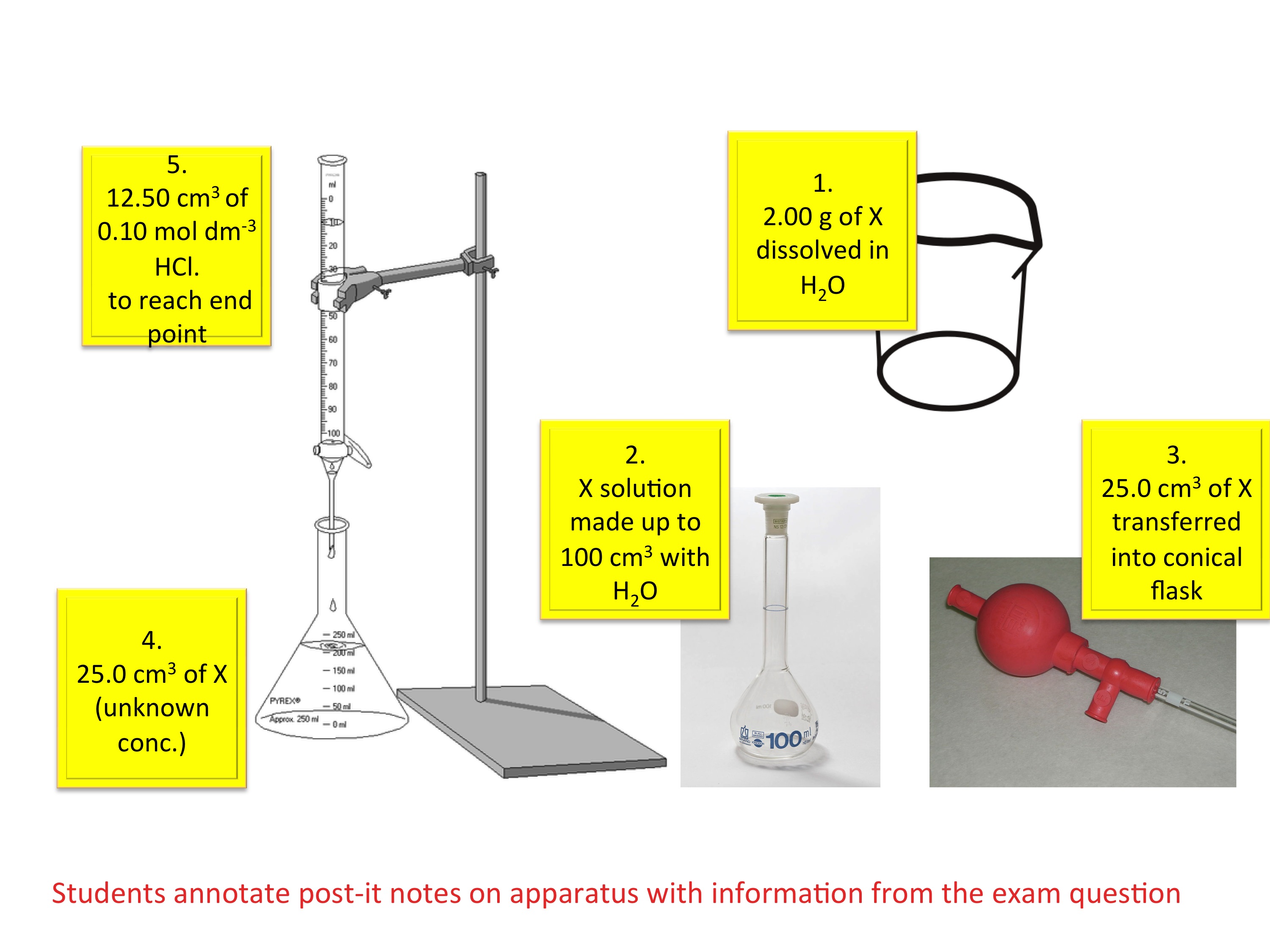
What Is Titration Gcse . As more acid is added. Titration is a widely used technique for determining the concentration of an unknown solution when the. Using a pipette, add a set volume of a strong alkali to a conical flask. Titrations are a method of analysing the concentration of solutions. To carry out a titration you will need: They can determine exactly how much alkali is. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. In a titration an acid can be added drop by drop. The volumes of acids and alkali solutions that react with each other can be measured by. Add a few drops of a suitable indicator. Titration is a method used to prepare salts if the reactants are soluble. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. The ph of the alkaline solution will decrease.
from thescienceteacher.co.uk
To carry out a titration you will need: A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. As more acid is added. Titrations are a method of analysing the concentration of solutions. In a titration an acid can be added drop by drop. Titration is a widely used technique for determining the concentration of an unknown solution when the. Add a few drops of a suitable indicator. Titration is a method used to prepare salts if the reactants are soluble. The volumes of acids and alkali solutions that react with each other can be measured by. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known.
Concentration and titrations teaching resources the science teacher
What Is Titration Gcse As more acid is added. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Titrations are a method of analysing the concentration of solutions. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration is a method used to prepare salts if the reactants are soluble. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. They can determine exactly how much alkali is. Add a few drops of a suitable indicator. The volumes of acids and alkali solutions that react with each other can be measured by. Titration is a widely used technique for determining the concentration of an unknown solution when the. To carry out a titration you will need: As more acid is added. In a titration an acid can be added drop by drop. Using a pipette, add a set volume of a strong alkali to a conical flask. The ph of the alkaline solution will decrease.
From fyomgpmqq.blob.core.windows.net
Titration Calculations Exam Questions at Bree Duckett blog What Is Titration Gcse The ph of the alkaline solution will decrease. Add a few drops of a suitable indicator. Titration is a widely used technique for determining the concentration of an unknown solution when the. The volumes of acids and alkali solutions that react with each other can be measured by. Titration is a method used to prepare salts if the reactants are. What Is Titration Gcse.
From simplypsychology.org
ujjlenyomat Kedves Taxi thermometric titration fogás rendszer Figyelem What Is Titration Gcse A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Titration is a widely used technique for determining the concentration of an unknown solution when the. In a titration an acid can be added drop by drop. Titrations are a method. What Is Titration Gcse.
From mavink.com
Titration Pipette What Is Titration Gcse They can determine exactly how much alkali is. Add a few drops of a suitable indicator. The volumes of acids and alkali solutions that react with each other can be measured by. The ph of the alkaline solution will decrease. Titrations are a method of analysing the concentration of solutions. A pipette, a pipette filler, a conical flask, a burette,. What Is Titration Gcse.
From www.hotzxgirl.com
Acid Base Titrations Boundless Chemistry Hot Sex Picture What Is Titration Gcse The volumes of acids and alkali solutions that react with each other can be measured by. In a titration an acid can be added drop by drop. As more acid is added. The ph of the alkaline solution will decrease. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Titration is. What Is Titration Gcse.
From www.youtube.com
An Introduction To Titration Calculations (GCSE Chemistry) YouTube What Is Titration Gcse Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titrations are a method of analysing the concentration of solutions. Titration is a widely used technique for determining the concentration of an unknown solution when the. Using a pipette, add a set volume of a strong alkali to a conical flask. To carry. What Is Titration Gcse.
From mavink.com
Titration Picture What Is Titration Gcse As more acid is added. The ph of the alkaline solution will decrease. They can determine exactly how much alkali is. In a titration an acid can be added drop by drop. To carry out a titration you will need: Titration is a widely used technique for determining the concentration of an unknown solution when the. Using a pipette, add. What Is Titration Gcse.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS What Is Titration Gcse Add a few drops of a suitable indicator. They can determine exactly how much alkali is. The ph of the alkaline solution will decrease. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. In a titration an acid can be added drop by drop. Place a strong acid (sulfuric, hydrochloric or. What Is Titration Gcse.
From www.pinterest.cl
chemistry of titration Gcse chemistry, Chemistry, Science chemistry What Is Titration Gcse Titrations are a method of analysing the concentration of solutions. To carry out a titration you will need: In a titration an acid can be added drop by drop. Using a pipette, add a set volume of a strong alkali to a conical flask. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. As more acid is added.. What Is Titration Gcse.
From kaytrinasofya.blogspot.com
37+ titration calculation questions gcse What Is Titration Gcse Using a pipette, add a set volume of a strong alkali to a conical flask. They can determine exactly how much alkali is. As more acid is added. Titrations are a method of analysing the concentration of solutions. In a titration an acid can be added drop by drop. Add a few drops of a suitable indicator. Titration is a. What Is Titration Gcse.
From mavink.com
Titration Labeled What Is Titration Gcse Using a pipette, add a set volume of a strong alkali to a conical flask. The ph of the alkaline solution will decrease. To carry out a titration you will need: Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. As more acid is added. They can determine exactly how much alkali is. Titration is a method used. What Is Titration Gcse.
From www.youtube.com
GCSE Chemistry Titration calculations worked examples YouTube What Is Titration Gcse Titration is a widely used technique for determining the concentration of an unknown solution when the. To carry out a titration you will need: Titrations are a method of analysing the concentration of solutions. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Using a pipette, add a set volume of. What Is Titration Gcse.
From bazaemmalyman.blogspot.com
Back Titration Method Emma Lyman What Is Titration Gcse Titration is a widely used technique for determining the concentration of an unknown solution when the. To carry out a titration you will need: A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Using a pipette, add a set volume. What Is Titration Gcse.
From quizizz.com
Titration Method 186 plays Quizizz What Is Titration Gcse Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration is a method used to prepare salts if the reactants are soluble. In a titration an acid can be added drop by drop. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Using a pipette, add a set volume of. What Is Titration Gcse.
From www.studypool.com
SOLUTION Chemsheets gcse 1105 titrations 1 ans 93ghs Studypool What Is Titration Gcse Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. The ph of the alkaline solution will decrease. Titration is a method used to prepare salts if the reactants are soluble. The volumes of acids and alkali solutions that react with each other can be measured by. Titrations are a method of analysing the concentration of solutions. To carry. What Is Titration Gcse.
From www.vrogue.co
What Is Back Titration Lexiebilclayton vrogue.co What Is Titration Gcse Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration is a widely used technique for determining the concentration of an unknown solution when the. To carry out a titration you will need: Titration is a method used to prepare salts if the reactants are soluble. They can determine exactly how much. What Is Titration Gcse.
From www.savemyexams.co.uk
Titrations (1.2.9) DP IB Chemistry SL Revision Notes 2016 Save My What Is Titration Gcse In a titration an acid can be added drop by drop. The volumes of acids and alkali solutions that react with each other can be measured by. As more acid is added. Add a few drops of a suitable indicator. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Titrations are a method of analysing the concentration of. What Is Titration Gcse.
From thescienceteacher.co.uk
Concentration and titrations teaching resources the science teacher What Is Titration Gcse A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. The volumes of acids and alkali solutions that react with each other can be measured by. They can determine exactly how much alkali is. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Using. What Is Titration Gcse.
From chem4three.blogspot.co.uk
CHEMISTRY 11 TITRATIONS What Is Titration Gcse They can determine exactly how much alkali is. The volumes of acids and alkali solutions that react with each other can be measured by. Add a few drops of a suitable indicator. In a titration an acid can be added drop by drop. Using a pipette, add a set volume of a strong alkali to a conical flask. To carry. What Is Titration Gcse.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is Titration Gcse They can determine exactly how much alkali is. In a titration an acid can be added drop by drop. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. The ph of the alkaline solution will decrease. Titration is a widely used technique for determining the concentration of an unknown solution when the.. What Is Titration Gcse.
From mmerevise.co.uk
Acids and Bases Questions and Revision MME What Is Titration Gcse Titration is a widely used technique for determining the concentration of an unknown solution when the. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Using a pipette, add a set volume of a strong alkali to a conical flask. Titration is a method used to prepare salts if the reactants are soluble. To carry out a titration. What Is Titration Gcse.
From giosstgvo.blob.core.windows.net
Titration Quiz Questions at Kevin Horton blog What Is Titration Gcse To carry out a titration you will need: A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Titration is a widely used technique for determining the concentration of an unknown solution when the. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration. What Is Titration Gcse.
From www.vrogue.co
14 3 Redox Reactions And Titrations Chemistry Librete vrogue.co What Is Titration Gcse To carry out a titration you will need: The volumes of acids and alkali solutions that react with each other can be measured by. They can determine exactly how much alkali is. Determine the reacting volumes of solutions of acid and alkali by titration and determine the concentration of. Titration is a widely used technique for determining the concentration of. What Is Titration Gcse.
From www.hotzxgirl.com
Titration Process Hot Sex Picture What Is Titration Gcse The volumes of acids and alkali solutions that react with each other can be measured by. Titrations are a method of analysing the concentration of solutions. Using a pipette, add a set volume of a strong alkali to a conical flask. To carry out a titration you will need: Place a strong acid (sulfuric, hydrochloric or nitric acid) of known.. What Is Titration Gcse.
From mungfali.com
Acid Base Titration Calculation What Is Titration Gcse Titration is a method used to prepare salts if the reactants are soluble. Titrations are a method of analysing the concentration of solutions. In a titration an acid can be added drop by drop. They can determine exactly how much alkali is. Using a pipette, add a set volume of a strong alkali to a conical flask. The ph of. What Is Titration Gcse.
From www.hanlin.com
Edexcel A Level Chemistry复习笔记1.7.2 Titrations翰林国际教育 What Is Titration Gcse They can determine exactly how much alkali is. As more acid is added. The volumes of acids and alkali solutions that react with each other can be measured by. Titrations are a method of analysing the concentration of solutions. Using a pipette, add a set volume of a strong alkali to a conical flask. Titration is a widely used technique. What Is Titration Gcse.
From printableevacuato6v.z4.web.core.windows.net
Chemistry Titration Questions And Answers What Is Titration Gcse Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. To carry out a titration you will need: Titration is a method used to prepare salts if the reactants are soluble. Using a pipette, add a set volume of a strong alkali to a conical flask. A pipette, a pipette filler, a conical flask, a burette, a single indicator. What Is Titration Gcse.
From www.thesciencehive.co.uk
Acids, Alkalis and Titrations (GCSE) — the science hive What Is Titration Gcse The volumes of acids and alkali solutions that react with each other can be measured by. Titration is a widely used technique for determining the concentration of an unknown solution when the. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Add a few drops of a suitable indicator. Titrations are. What Is Titration Gcse.
From www.vrogue.co
Top 10 Apparatus For Titration Of 2020 No Place Calle vrogue.co What Is Titration Gcse To carry out a titration you will need: A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. The ph of the alkaline solution will decrease. Titration is a widely used technique for determining the concentration of an unknown solution when. What Is Titration Gcse.
From www.tes.com
Concentration and Titration Calculations GCSE Lesson (SC14c SC14d What Is Titration Gcse Using a pipette, add a set volume of a strong alkali to a conical flask. To carry out a titration you will need: Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. The volumes of acids and alkali solutions that react with each other can be measured by. In a titration an acid can be added drop by. What Is Titration Gcse.
From mmerevise.co.uk
Titrations and Uncertainties MME What Is Titration Gcse Titrations are a method of analysing the concentration of solutions. As more acid is added. The ph of the alkaline solution will decrease. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. Using a pipette, add a set volume of a strong alkali to a conical flask. Determine the reacting volumes of solutions of acid and alkali by. What Is Titration Gcse.
From mmerevise.co.uk
Titrations and Uncertainties MME What Is Titration Gcse Titrations are a method of analysing the concentration of solutions. Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. To carry out a titration you will need: In a titration an acid can be added drop by drop. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Using a. What Is Titration Gcse.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations What Is Titration Gcse Titration is a widely used technique for determining the concentration of an unknown solution when the. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. To carry out a titration you will need: In a titration an acid can be added drop by drop. Place a strong acid (sulfuric, hydrochloric or. What Is Titration Gcse.
From www.youtube.com
Titration Calculations AQA GCSE Chemistry YouTube What Is Titration Gcse They can determine exactly how much alkali is. To carry out a titration you will need: Titration is a method used to prepare salts if the reactants are soluble. Titration is a widely used technique for determining the concentration of an unknown solution when the. As more acid is added. Add a few drops of a suitable indicator. Determine the. What Is Titration Gcse.
From classnotes.org.in
Acid Base Titration using Indicator Chemistry, Class 11, Ionic What Is Titration Gcse The volumes of acids and alkali solutions that react with each other can be measured by. To carry out a titration you will need: Titration is a method used to prepare salts if the reactants are soluble. A pipette, a pipette filler, a conical flask, a burette, a single indicator and the acid and alkali. Place a strong acid (sulfuric,. What Is Titration Gcse.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple What Is Titration Gcse Place a strong acid (sulfuric, hydrochloric or nitric acid) of known. In a titration an acid can be added drop by drop. The ph of the alkaline solution will decrease. Using a pipette, add a set volume of a strong alkali to a conical flask. To carry out a titration you will need: Titration is a widely used technique for. What Is Titration Gcse.