Aspirin Acetaminophen Polarity . Fill the flask to the. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Analysis of aspirin”, by houston byrd and stephen e. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask.
from www.numerade.com
Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Fill the flask to the. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen.
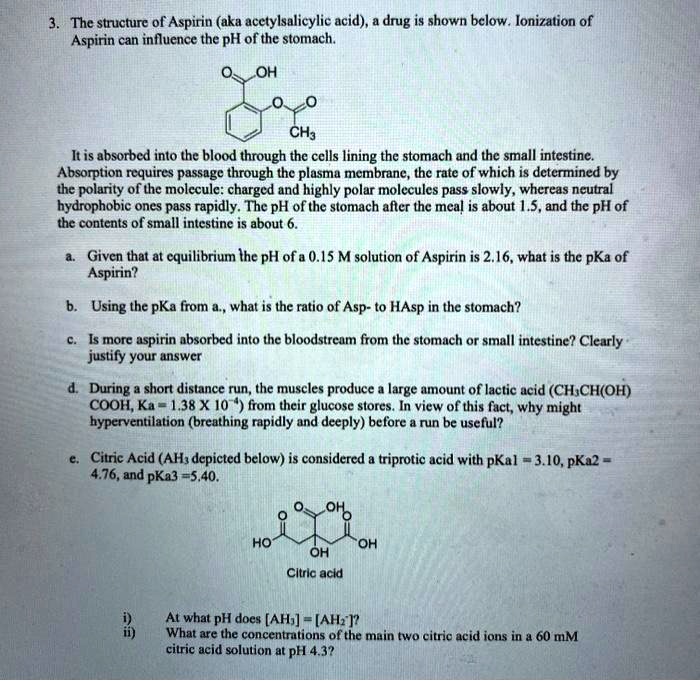
SOLVED The structure of Aspirin (aka acetylsalicylic acid), a drug, is
Aspirin Acetaminophen Polarity Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Fill the flask to the. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18.
From brownemblog.com
Acetaminophen, Acetylcysteine, and Anaphylaxis With a Twist — BROWN Aspirin Acetaminophen Polarity Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Analysis of aspirin”, by houston byrd and stephen e. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Fill the flask to the. Aspirin, acetaminophen, and caffeine separation from excedrin via. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED Rank the polarity of following compounds Aspirin, caffeine Aspirin Acetaminophen Polarity With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. The purity of the materials (98.07 mol% for aspirin, 99.98 mol%. Aspirin Acetaminophen Polarity.
From slideplayer.com
AP Chemistry bellringers ppt download Aspirin Acetaminophen Polarity The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into. Aspirin Acetaminophen Polarity.
From www.youtube.com
Aspirin to Acetaminophen Part 6 of 6 Acetaminophen from p Aspirin Acetaminophen Polarity Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. The purity of the materials (98.07. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved Please help for 8 The 5 analgesics mentioned in the Aspirin Acetaminophen Polarity Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Fill the flask to the. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED Lab Extraction and Isolation of Caffeine and Aspirin Explain Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Fill the flask to the. Using a pipet, transfer 10 ml of the acetaminophen solution into. Aspirin Acetaminophen Polarity.
From www.indiamart.com
pharmaceutical tablets Acetaminophen Aspirin Tablet Retailer from Angul Aspirin Acetaminophen Polarity Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. The purity of. Aspirin Acetaminophen Polarity.
From thecontentauthority.com
Acetaminophen vs Aspirin Differences And Uses For Each One Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Acetaminophen, aspirin and. Aspirin Acetaminophen Polarity.
From www.dreamstime.com
Aspirin Ibuprofen Acetaminophen Stock Photo Image of fever, dose Aspirin Acetaminophen Polarity Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Analysis of aspirin”, by houston. Aspirin Acetaminophen Polarity.
From acetaminophenrules.blogspot.com
Acetaminophen A Miracle Worker Acetaminophen And It's Polarity Aspirin Acetaminophen Polarity Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Fill the flask to the. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Analysis of aspirin”, by houston byrd. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved 2) The structures of caffeine, aspirin, and Tylenol Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Fill the flask to. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved Out of aspirin, ibuprofen caffeine, and Aspirin Acetaminophen Polarity Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine. Aspirin Acetaminophen Polarity.
From hirata.softsync.jp
連載 22日目 アセトアミノフェンについて深く知ろう④ 平田の薬剤師塾 ~薬のことを分かりやすく丁寧に~ Aspirin Acetaminophen Polarity Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. With the addition. Aspirin Acetaminophen Polarity.
From www.chegg.com
Chemistry Archive March 08, 2017 Aspirin Acetaminophen Polarity Fill the flask to the. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Analysis of aspirin”, by houston byrd and stephen e. Learn how to use flash column chromatography to. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED acetaminophen, aspirin, caffeine predict which compound will be Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal. Aspirin Acetaminophen Polarity.
From askanydifference.com
アセトアミノフェンとアスピリン 違いと比較 Aspirin Acetaminophen Polarity Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Analysis of aspirin”, by houston byrd and stephen e. Acetaminophen, aspirin and. Aspirin Acetaminophen Polarity.
From www.numerade.com
Rank the polarity of following compounds Aspirin, caffeine Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. The purity of the materials (98.07 mol% for. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved Rank Aspirin Acetaminophen Caffine phenacetin Aspirin Acetaminophen Polarity The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Fill the flask to the. Using a pipet, transfer 10 ml of the acetaminophen solution into a. Aspirin Acetaminophen Polarity.
From askanydifference.com
アセトアミノフェンとアスピリン 違いと比較 Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask.. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved Aspirin (acid) And KOH (base) D. Acetaminophen (ac... Aspirin Acetaminophen Polarity Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Analysis of aspirin”, by houston byrd and stephen e. The purity of the materials (98.07. Aspirin Acetaminophen Polarity.
From www.alamy.com
Acetaminophen Black and White Stock Photos & Images Alamy Aspirin Acetaminophen Polarity Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Fill the flask to the. With the addition of caffeine, the painkilling effect can. Aspirin Acetaminophen Polarity.
From www.sielc.com
Acetaminophen (Paracetamol) SIELC Aspirin Acetaminophen Polarity Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Analysis of aspirin”, by houston byrd and stephen e. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Fill the flask to the. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: With the addition of. Aspirin Acetaminophen Polarity.
From byjus.com
Aspirin is an acetylation product of Aspirin Acetaminophen Polarity Fill the flask to the. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100. Aspirin Acetaminophen Polarity.
From www.chegg.com
A Student Extracted The Active Ingredient From Sev... Aspirin Acetaminophen Polarity The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Fill the flask to the. Analysis of aspirin”, by houston byrd and stephen e. With the addition. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED Is salicylic acid less polar than acetaminophen? If so, why? Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Analysis of aspirin”, by houston byrd and stephen e. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83. Aspirin Acetaminophen Polarity.
From www.chegg.com
Solved Acetaminophen Acetylsalicylic Acid Caffeine Aspirin Acetaminophen Polarity With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under. Aspirin Acetaminophen Polarity.
From fda.report
ACETAMINOPHEN 250MG ASPIRIN 250MG CAFFEINE 65MG tablet Aspirin Acetaminophen Polarity Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. With the addition. Aspirin Acetaminophen Polarity.
From www.dreamstime.com
3D Image of Aspirin Skeletal Formula Stock Illustration Illustration Aspirin Acetaminophen Polarity With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Aspirin, acetaminophen, and caffeine separation. Aspirin Acetaminophen Polarity.
From www.guidechem.com
Acetaminophen Chemical Dictionary Guidechem Aspirin Acetaminophen Polarity Analysis of aspirin”, by houston byrd and stephen e. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and. Aspirin Acetaminophen Polarity.
From studylib.net
Synthesis of Aspirin and Acetaminophen Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Analysis of aspirin”, by houston byrd and stephen e. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Fill the flask to. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED The structure of Aspirin (aka acetylsalicylic acid), a drug, is Aspirin Acetaminophen Polarity Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Analysis of aspirin”, by houston byrd and stephen e. The purity of the. Aspirin Acetaminophen Polarity.
From www.freepik.com
Premium Vector Aspirin chemistry chemical formula structure vector Aspirin Acetaminophen Polarity Fill the flask to the. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: The purity of the materials (98.07 mol% for aspirin, 99.98 mol% for caeine and 99.83 mol% for paracetamol) was determined by differential. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Using a pipet, transfer 10 ml of. Aspirin Acetaminophen Polarity.
From www.numerade.com
SOLVED Q2) List the following compounds from least polar to most polar Aspirin Acetaminophen Polarity Analysis of aspirin”, by houston byrd and stephen e. Fill the flask to the. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Learn. Aspirin Acetaminophen Polarity.
From slideplayer.com
Week 6 TLC of Analgesics Today’s Agenda Analgesics Polarity ppt download Aspirin Acetaminophen Polarity Analysis of aspirin”, by houston byrd and stephen e. Aspirin, acetaminophen, and caffeine separation from excedrin via column chromatography introduction: Acetaminophen, aspirin and caffeine have been successfully analyzed with an internal standard (benzoic acid) in under six minutes using a c18. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Fill the flask. Aspirin Acetaminophen Polarity.
From athome.medline.com
Medline Acetaminophen / Aspirin / Caffeine Tablet 100Ct Aspirin Acetaminophen Polarity With the addition of caffeine, the painkilling effect can be intensified with analgesics like acetaminophen, aspirin, and ibuprofen. Using a pipet, transfer 10 ml of the acetaminophen solution into a 100 ml volumetric flask. Learn how to use flash column chromatography to separate aspirin, acetaminophen, and caffeine from excedrin®. Fill the flask to the. Acetaminophen, aspirin and caffeine have been. Aspirin Acetaminophen Polarity.