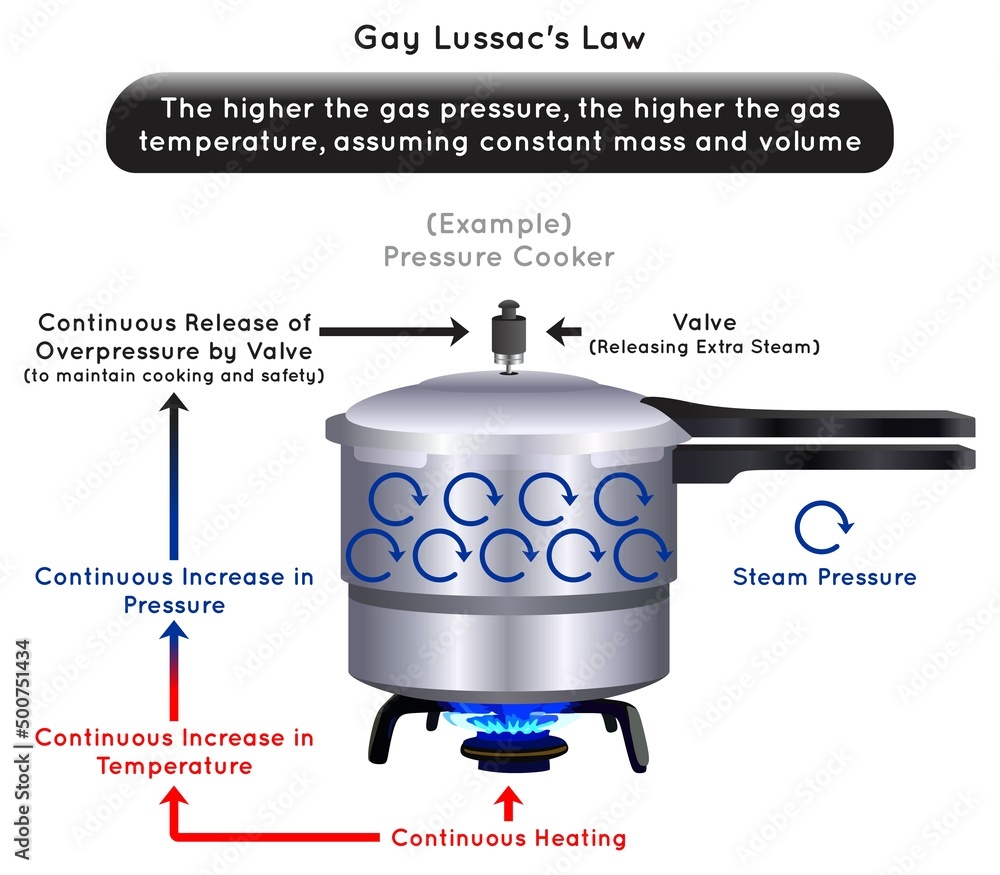
How Do Pressure Cookers Work Chemistry . Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. construction and function of a pressure cooker. the science behind pressure cookers. the benefits of cooking food using a pressure cooker. time for a quick high school chemistry refresher: The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. It is commonly given as: how do pressure cookers work?
from ar.inspiredpencil.com
the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. the science behind pressure cookers. It is commonly given as: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. the benefits of cooking food using a pressure cooker. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. time for a quick high school chemistry refresher: construction and function of a pressure cooker. how do pressure cookers work?
Pressure Cooker Diagram
How Do Pressure Cookers Work Chemistry the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. It is commonly given as: how do pressure cookers work? the benefits of cooking food using a pressure cooker. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. the science behind pressure cookers. time for a quick high school chemistry refresher: construction and function of a pressure cooker. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e.
From www.gaomon.com
How Does a Pressure Cooker Work? How Do Pressure Cookers Work Chemistry the science behind pressure cookers. the benefits of cooking food using a pressure cooker. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. time for a quick high school chemistry refresher: how do pressure cookers work? the key. How Do Pressure Cookers Work Chemistry.
From www.prestige.co.uk
How Do Pressure Cookers Work? A Complete Guide by Prestige How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: how do pressure cookers work? Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. construction and function of a pressure cooker. . How Do Pressure Cookers Work Chemistry.
From www.linkedin.com
How do pressure cookers work? How Do Pressure Cookers Work Chemistry the science behind pressure cookers. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. It is commonly given as: time for a quick high school chemistry refresher: how do pressure cookers work? Now that you understand the basics of boiling. How Do Pressure Cookers Work Chemistry.
From www.thespruceeats.com
How Do Pressure Cookers Work? The Science Behind Them How Do Pressure Cookers Work Chemistry construction and function of a pressure cooker. It is commonly given as: the benefits of cooking food using a pressure cooker. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the science behind pressure cookers. the key scientific principle at play in pressure cooking is the direct relationship between. How Do Pressure Cookers Work Chemistry.
From ovenspot.com
How do Pressure Cookers Work? Pressure Cooking 101 How Do Pressure Cookers Work Chemistry construction and function of a pressure cooker. It is commonly given as: the benefits of cooking food using a pressure cooker. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Now that you understand the basics of boiling and its relation. How Do Pressure Cookers Work Chemistry.
From www.housejunkie.co.uk
How Does a Pressure Cooker Work? The Science Behind It House Junkie How Do Pressure Cookers Work Chemistry It is commonly given as: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the benefits of cooking food using a pressure cooker. the science behind pressure cookers. construction and function of a pressure cooker. how do pressure cookers work? The pressure cooker can be best explained by the. How Do Pressure Cookers Work Chemistry.
From ar.inspiredpencil.com
Pressure Cooker Diagram How Do Pressure Cookers Work Chemistry the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. how do pressure cookers work? It is. How Do Pressure Cookers Work Chemistry.
From juliannakunstler.com
How Does A Pressure Cooker Work Chemistry Discount How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. It is commonly given as: how do pressure cookers work? The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. construction and function of a pressure cooker. the science behind pressure. How Do Pressure Cookers Work Chemistry.
From missvickie.com
How Does A Pressure Cooker Work? Miss Vickie How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. construction and function of a pressure cooker. how do pressure cookers work? the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the. How Do Pressure Cookers Work Chemistry.
From dxoogmceb.blob.core.windows.net
How To Know Pressure Cooker Is Working at Art Anderson blog How Do Pressure Cookers Work Chemistry the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. how do pressure cookers work? The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. time. How Do Pressure Cookers Work Chemistry.
From www.youtube.com
How a Pressure Cooker Works Working of Pressure Cooker Science How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. construction and function of a pressure cooker. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics.. How Do Pressure Cookers Work Chemistry.
From www.vrogue.co
How Do Pressure Cookers Work Infographic Work Infogra vrogue.co How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. time for a quick high school chemistry refresher: construction and function of a pressure cooker. It is commonly given as: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the science behind pressure cookers. how do pressure cookers work?. How Do Pressure Cookers Work Chemistry.
From staah-cloud.com
How do pressure cookers work? A complete guide by Prestige / Prestige How Do Pressure Cookers Work Chemistry the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. time for a quick high school chemistry refresher: The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most. How Do Pressure Cookers Work Chemistry.
From www.best-infographics.com
How Do Pressure Cookers Work [Infographic] Best Infographics How Do Pressure Cookers Work Chemistry construction and function of a pressure cooker. It is commonly given as: time for a quick high school chemistry refresher: Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the. How Do Pressure Cookers Work Chemistry.
From www.hippressurecooking.com
How The Pressure Cooker Works Pressure Cooking School hip pressure How Do Pressure Cookers Work Chemistry the science behind pressure cookers. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. construction and function of a pressure cooker. the benefits of cooking food using a pressure cooker. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”),. How Do Pressure Cookers Work Chemistry.
From www.vlr.eng.br
How Do Pressure Cooker Work vlr.eng.br How Do Pressure Cookers Work Chemistry construction and function of a pressure cooker. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. time for a quick high school chemistry refresher: The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which. How Do Pressure Cookers Work Chemistry.
From www.tffn.net
How Does a Pressure Cooker Work? A Comprehensive Guide The How Do Pressure Cookers Work Chemistry the science behind pressure cookers. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. time for a quick high school chemistry refresher: Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at. How Do Pressure Cookers Work Chemistry.
From stravaganzastravaganza.blogspot.com
S T R A V A G A N Z A HISTORY OF PRESSURE COOKER How Do Pressure Cookers Work Chemistry It is commonly given as: time for a quick high school chemistry refresher: Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. construction and function of a pressure cooker. how do pressure cookers work? the benefits of cooking food using a pressure cooker. Normally water. How Do Pressure Cookers Work Chemistry.
From www.thespruceeats.com
How Do Pressure Cookers Work? The Science Behind Them How Do Pressure Cookers Work Chemistry the science behind pressure cookers. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. construction and function of a pressure cooker. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the benefits of. How Do Pressure Cookers Work Chemistry.
From www.pressurecookrecipes.com
How to Use a Pressure Cooker Simple Guide by Amy + Jacky How Do Pressure Cookers Work Chemistry Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. the benefits of cooking food using a pressure cooker. It is commonly given as: The pressure cooker can be best explained by the. How Do Pressure Cookers Work Chemistry.
From www.scienceabc.com
What Is A Pressure Cooker? How Does A Pressure Cooker Work? How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: construction and function of a pressure cooker. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. . How Do Pressure Cookers Work Chemistry.
From www.youtube.com
Science of Pressure Cooking How Pressure Cookers Work YouTube How Do Pressure Cookers Work Chemistry The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. the key scientific principle at play in pressure cooking is the direct relationship. How Do Pressure Cookers Work Chemistry.
From www.youtube.com
How does the Pressure cooker works? YouTube How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. It is commonly given as: The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. how do. How Do Pressure Cookers Work Chemistry.
From www.tffn.net
How Does a Pressure Cooker Work? Exploring the Physics and Benefits How Do Pressure Cookers Work Chemistry the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. the benefits of cooking food using a pressure cooker. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. construction and function. How Do Pressure Cookers Work Chemistry.
From www.youtube.com
How pressure cooker works/science behind pressure cooker/parts of How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. construction and function of. How Do Pressure Cookers Work Chemistry.
From es.slideshare.net
C24 the chemistry of cooking How Do Pressure Cookers Work Chemistry Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. time for a quick high school chemistry refresher: It is commonly given as: how do pressure cookers work? the key scientific. How Do Pressure Cookers Work Chemistry.
From www.best-infographics.com
How Do Pressure Cookers Work [Infographic] Best Infographics How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. It is commonly given. How Do Pressure Cookers Work Chemistry.
From www.youtube.com
How does a pressure cooker work? Explained/UPSC Question (GS PODCAST How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. It is commonly given as: Now that you understand the basics of boiling and its relation with pressure, let’s take a closer look at the.. How Do Pressure Cookers Work Chemistry.
From mychefscookware.com
What Is a Pressure Cooker and How Does It Really Work? My Chefs Cookware How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. construction and function of a pressure cooker. the benefits of cooking food using a pressure cooker. the key scientific principle at play in pressure cooking is the direct relationship between pressure and. How Do Pressure Cookers Work Chemistry.
From botw.org
How Do Pressure Cookers and Instant Pots Work? How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most. How Do Pressure Cookers Work Chemistry.
From www.thespruceeats.com
Pressure Cooker Basics How Do Pressure Cookers Work Chemistry the benefits of cooking food using a pressure cooker. the science behind pressure cookers. the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. Now that. How Do Pressure Cookers Work Chemistry.
From exoeilfrq.blob.core.windows.net
How To Turn On My Pressure Cooker at Eleanor Welch blog How Do Pressure Cookers Work Chemistry Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. how do pressure cookers work? the key scientific principle at play in pressure cooking is the direct relationship between pressure and temperature, described in the ideal gas law in thermodynamics. the benefits of cooking food using a pressure cooker. time. How Do Pressure Cookers Work Chemistry.
From www.tffn.net
How Does a Pressure Cooker Work? Exploring the Physics and Benefits How Do Pressure Cookers Work Chemistry time for a quick high school chemistry refresher: It is commonly given as: the benefits of cooking food using a pressure cooker. the science behind pressure cookers. Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. how do pressure cookers work? The pressure cooker can be best explained by. How Do Pressure Cookers Work Chemistry.
From www.marthastewart.com
A Simple Explanation of How Pressure Cookers Work Martha Stewart How Do Pressure Cookers Work Chemistry It is commonly given as: the benefits of cooking food using a pressure cooker. construction and function of a pressure cooker. time for a quick high school chemistry refresher: Normally water will boil at 212f (100c) at sea level atmospheric pressure at 1,01325 bar, i.e. how do pressure cookers work? the science behind pressure cookers.. How Do Pressure Cookers Work Chemistry.
From www.hippressurecooking.com
The Pressure Cooker's Parts Pressure Cooking School ⋆ hip pressure How Do Pressure Cookers Work Chemistry It is commonly given as: the science behind pressure cookers. construction and function of a pressure cooker. The pressure cooker can be best explained by the “ideal gas law” (or “general gas equation”), which describes the behavior of most gases under most conditions. time for a quick high school chemistry refresher: Now that you understand the basics. How Do Pressure Cookers Work Chemistry.