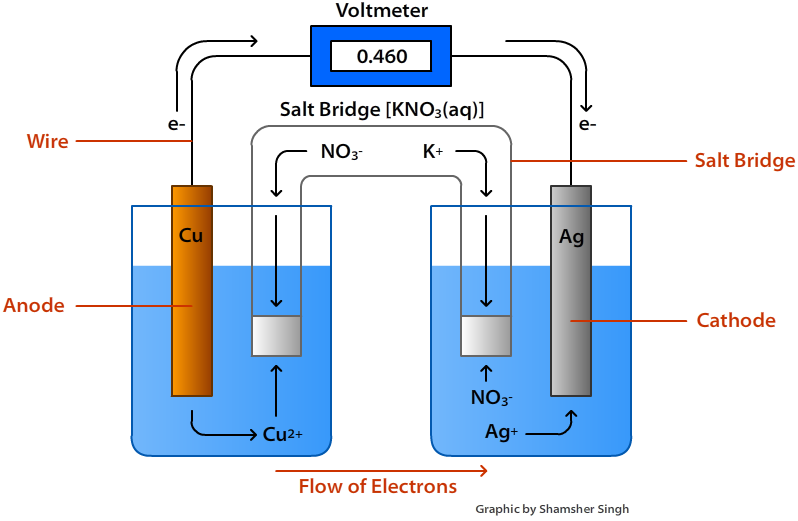
Type Of Electrical Source Of Electrochemical And Example . Galvanic cells use a chemical reaction, usually a redox. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Both types contain two electrodes , one anode and one cathode. Electrochemical cells use an applied source of energy to produce a chemical reaction; There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction.
from chem.libretexts.org
Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Both types contain two electrodes , one anode and one cathode. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells use an applied source of energy to produce a chemical reaction; There are two types of electrochemical cells: Galvanic cells use a chemical reaction, usually a redox.
Voltaic Cells Chemistry LibreTexts
Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Galvanic cells use a chemical reaction, usually a redox. There are two types of electrochemical cells: Electrochemical cells use an applied source of energy to produce a chemical reaction; Both types contain two electrodes , one anode and one cathode. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well.
From proper-cooking.info
Electrochemical Energy Type Of Electrical Source Of Electrochemical And Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Both types contain two electrodes , one anode and one cathode. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of the. Type Of Electrical Source Of Electrochemical And Example.
From study.com
Electrochemical Cell Definition, Types & Examples Lesson Type Of Electrical Source Of Electrochemical And Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells use a chemical reaction, usually a redox. There are two types of electrochemical cells: Galvanic. Type Of Electrical Source Of Electrochemical And Example.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells (also called voltaic cells) and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Both types contain two electrodes , one anode and one cathode. Electrochemical cells, also. Type Of Electrical Source Of Electrochemical And Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Both types contain two electrodes , one anode and one cathode. Galvanic cells (also called voltaic cells) and electrolytic cells. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemistry is the study of the generation. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Simplified outline for electrochemical method Download Scientific Diagram Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells. There are two types of electrochemical cells: Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Scheme of an experimental 3 electrode electrochemical cell. Download Type Of Electrical Source Of Electrochemical And Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells use a chemical reaction, usually a redox. Galvanic cells (also called voltaic cells) and electrolytic cells. Both types contain two electrodes , one anode and one. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Electrochemical equivalent circuit diagram Download HighQuality Type Of Electrical Source Of Electrochemical And Example Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. There are two types of electrochemical cells: Both types contain two electrodes , one anode and one cathode. Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells (also called voltaic cells) and electrolytic cells. An. Type Of Electrical Source Of Electrochemical And Example.
From www.mr-electric.co.uk
Different Types of Electrical Sources Mr Electric Blog Type Of Electrical Source Of Electrochemical And Example Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. There are two types of electrochemical cells: Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely,. Type Of Electrical Source Of Electrochemical And Example.
From slidetodoc.com
Types of Electrochemical Cells n n Electrolytic Cells Type Of Electrical Source Of Electrochemical And Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemistry is the study of the generation of electricity from the energy released during. Type Of Electrical Source Of Electrochemical And Example.
From mavink.com
Diagram Of Electrochemical Cell Type Of Electrical Source Of Electrochemical And Example Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells use an applied source of energy to produce a chemical reaction; There are two types of electrochemical cells: Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells use a chemical reaction, usually a redox. An apparatus that. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
1. (a) A schematic diagram of electrochemical double layer, (b) the Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. There are two types of electrochemical cells: Galvanic cells (also called voltaic cells) and electrolytic cells. Galvanic cells use a chemical reaction, usually. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Design of electrochemical ozone generator with oxygen cathode [25 Type Of Electrical Source Of Electrochemical And Example Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells use an applied source of energy to produce a chemical reaction; Both types contain two electrodes , one anode and one cathode. Galvanic cells use a chemical reaction, usually a. Type Of Electrical Source Of Electrochemical And Example.
From alevelchemistry.co.uk
Electrochemical Cells Definition, Description & Types Type Of Electrical Source Of Electrochemical And Example Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Electrochemical cells use an applied source of energy to produce a chemical reaction; An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Galvanic cells use a. Type Of Electrical Source Of Electrochemical And Example.
From mrselliott.wordpress.com
Electrochemistry, featuring electrolysis and fuel cells. mrs elliott Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells use an applied source of energy to produce a chemical reaction; Both types contain two electrodes , one anode and one. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
17. A schematic view of electrochemical setup 3electrode cell in a Type Of Electrical Source Of Electrochemical And Example Galvanic cells (also called voltaic cells) and electrolytic cells. There are two types of electrochemical cells: Galvanic cells use a chemical reaction, usually a redox. Both types contain two electrodes , one anode and one cathode. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Electrochemical cells use an applied. Type Of Electrical Source Of Electrochemical And Example.
From www.sigmaaldrich.com
Electrochemistry on the Bench and in the Field Type Of Electrical Source Of Electrochemical And Example Both types contain two electrodes , one anode and one cathode. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Galvanic cells use a chemical reaction, usually a redox. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. Type Of Electrical Source Of Electrochemical And Example.
From www.youtube.com
9.4.1 Explain how a redox reaction is used to produce electricity in a Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells use a chemical reaction, usually a redox. Galvanic cells (also called voltaic cells) and electrolytic cells. There are two types of electrochemical cells: Both types contain. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Diagram of a three electrode electrochemical cell. Download Type Of Electrical Source Of Electrochemical And Example Galvanic cells (also called voltaic cells) and electrolytic cells. Both types contain two electrodes , one anode and one cathode. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells use a chemical reaction, usually a redox. There are two types of electrochemical cells: Electrochemistry is the study of. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Classification of electrochemical cells [26] Download Scientific Diagram Type Of Electrical Source Of Electrochemical And Example Galvanic cells (also called voltaic cells) and electrolytic cells. Both types contain two electrodes , one anode and one cathode. Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells use a chemical reaction, usually a. Type Of Electrical Source Of Electrochemical And Example.
From slidetodoc.com
Basic Concepts of Electrochemistry ELECTROCHEMISTRY Electricitydriven Type Of Electrical Source Of Electrochemical And Example There are two types of electrochemical cells: Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. An apparatus. Type Of Electrical Source Of Electrochemical And Example.
From www.slideserve.com
PPT Types of Electrochemical Cells PowerPoint Presentation, free Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Electrochemical cells use an applied source of energy to produce a chemical reaction; Both types contain two electrodes , one anode and one cathode. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic. Type Of Electrical Source Of Electrochemical And Example.
From www.geeksforgeeks.org
Electrochemical Cell Definition, Types, Working Principle & Uses Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Both types contain two electrodes , one anode. Type Of Electrical Source Of Electrochemical And Example.
From slideplayer.com
Electrochemistry. ppt download Type Of Electrical Source Of Electrochemical And Example Galvanic cells use a chemical reaction, usually a redox. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. There are two types of electrochemical cells: Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy. Type Of Electrical Source Of Electrochemical And Example.
From www.slideserve.com
PPT Types of Electrochemical Cells PowerPoint Presentation, free Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells.. Type Of Electrical Source Of Electrochemical And Example.
From philschatz.com
Electrolysis · Chemistry Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. There are two types of electrochemical cells: Both types contain two electrodes , one anode and one cathode. Electrochemistry. Type Of Electrical Source Of Electrochemical And Example.
From saylordotorg.github.io
Describing Electrochemical Cells Type Of Electrical Source Of Electrochemical And Example Both types contain two electrodes , one anode and one cathode. Galvanic cells use a chemical reaction, usually a redox. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. An apparatus that is used to generate electricity from a spontaneous redox. Type Of Electrical Source Of Electrochemical And Example.
From saylordotorg.github.io
Electrochemistry Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Galvanic cells use a chemical reaction, usually a redox. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. There are two types of electrochemical cells: An apparatus that is used to generate electricity from a spontaneous redox. Type Of Electrical Source Of Electrochemical And Example.
From www.yaclass.in
Types of Electrochemical Cell and Electrolytic Cell — lesson. Science Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Both types contain two electrodes , one anode and one cathode. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses. Type Of Electrical Source Of Electrochemical And Example.
From chem.libretexts.org
Voltaic Cells Chemistry LibreTexts Type Of Electrical Source Of Electrochemical And Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells. Galvanic cells use a chemical reaction, usually a redox. There are two types. Type Of Electrical Source Of Electrochemical And Example.
From saylordotorg.github.io
Electrochemistry Type Of Electrical Source Of Electrochemical And Example Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells use a chemical reaction, usually a redox. An apparatus that is used to generate electricity from a. Type Of Electrical Source Of Electrochemical And Example.
From mungfali.com
Electrolysis Process Diagram Type Of Electrical Source Of Electrochemical And Example Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. There are two types of electrochemical cells: Electrochemical cells use an applied source of energy to produce a chemical reaction; Both types contain two electrodes , one anode and one cathode. Galvanic cells (also called voltaic cells) and electrolytic cells. An. Type Of Electrical Source Of Electrochemical And Example.
From www.researchgate.net
Illustration of the electrochemical CO2 reduction process. Download Type Of Electrical Source Of Electrochemical And Example Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. There are two types of electrochemical cells: Electrochemical cells use an applied source of energy to produce a chemical reaction; An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a. Type Of Electrical Source Of Electrochemical And Example.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Type Of Electrical Source Of Electrochemical And Example Both types contain two electrodes , one anode and one cathode. Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. Galvanic cells (also called voltaic cells) and electrolytic cells. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. There are. Type Of Electrical Source Of Electrochemical And Example.
From sciencevision.in
Electrolytes , Electolytic Cell And Electrochemical Cell Science Vision Type Of Electrical Source Of Electrochemical And Example An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells, also known as galvanic cells or voltaic cells, are devices that convert chemical energy into electrical energy. Galvanic cells use a chemical reaction, usually a redox. Galvanic cells (also called voltaic cells) and electrolytic. Type Of Electrical Source Of Electrochemical And Example.
From studylib.net
Electrochemical cells Type Of Electrical Source Of Electrochemical And Example Electrochemical cells use an applied source of energy to produce a chemical reaction; Electrochemistry is the study of the generation of electricity from the energy released during spontaneous chemical reactions, as well. An apparatus that is used to generate electricity from a spontaneous redox reaction or, conversely, that uses electricity to drive a nonspontaneous redox reaction. Electrochemical cells, also known. Type Of Electrical Source Of Electrochemical And Example.