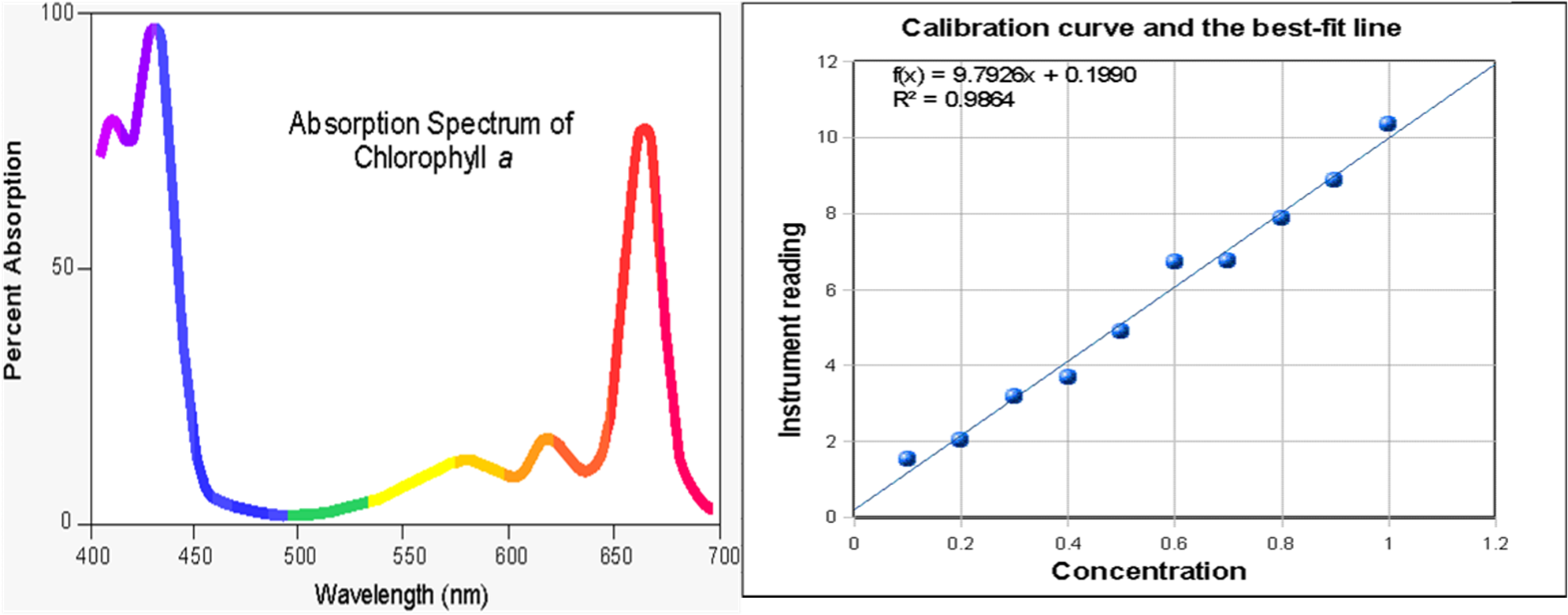
Wavelength And Absorbance Relationship . As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Compare the data for the mixture with the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. First the class is divided into groups of 3 or 4 and the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because
from public.websites.umich.edu
First the class is divided into groups of 3 or 4 and the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Compare the data for the mixture with the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration.
Chem 125 Experiment II
Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. First the class is divided into groups of 3 or 4 and the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. Compare the data for the mixture with the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution?
From www.superprof.fr
L'Absorbance Superprof Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. In this experiment students are asked to discover the relationship between color,. Wavelength And Absorbance Relationship.
From www.youtube.com
How to estimate the size of nanoparticles from UVVis absorbance in Wavelength And Absorbance Relationship Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Compare the data for the mixture with. Wavelength And Absorbance Relationship.
From www.researchgate.net
Graph of relationship between absorbance value and wavelength of dye DN Wavelength And Absorbance Relationship As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because First the class is divided into groups of 3 or 4 and the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and. Wavelength And Absorbance Relationship.
From www.researchgate.net
The relationship between absorbance and wavelength of Wavelength And Absorbance Relationship In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. First the class is divided into groups of 3 or 4 and the. Compare the data for the mixture with the. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l. Wavelength And Absorbance Relationship.
From www.researchgate.net
a Absorbance curves at wavelengths of 300800 nm for 200 min, b graph Wavelength And Absorbance Relationship Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. First the class is divided into groups of 3 or 4 and the. Q 1:what relationship exists between the. Wavelength And Absorbance Relationship.
From www.researchgate.net
relationship between absorbance and wavelengths of MgO samples shows Wavelength And Absorbance Relationship Compare the data for the mixture with the. First the class is divided into groups of 3 or 4 and the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures. Wavelength And Absorbance Relationship.
From www.researchgate.net
Calibration of spectrophotometer by measuring absorbance of CuSO4 Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. Compare the data for the mixture with the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and. Wavelength And Absorbance Relationship.
From www.researchgate.net
Relationship between absorbance and wavelength of AFGMWCNT nanofluids Wavelength And Absorbance Relationship Compare the data for the mixture with the. First the class is divided into groups of 3 or 4 and the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because In this. Wavelength And Absorbance Relationship.
From theoryanalysis.netlify.app
Relation between particle size and absorbance Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a. Wavelength And Absorbance Relationship.
From www.researchgate.net
The relationship between absorbance and wavelength of Wavelength And Absorbance Relationship As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε),. Wavelength And Absorbance Relationship.
From einvoice.fpt.com.vn
SOLVED Based On The Graph Of Absorbance Versus Wavelength, 58 OFF Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because First the class is divided into groups of 3 or 4 and the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. As you likely know from other experiences, a particular chemical. Wavelength And Absorbance Relationship.
From www.researchgate.net
Normalized absorbance and emission intensity spectra versus wavelength Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? First the class is divided into groups of 3 or 4 and the. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. In this experiment students. Wavelength And Absorbance Relationship.
From definitionhjo.blogspot.com
Definition Of Absorbance In Chemistry DEFINITION HJO Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Compare the data for the mixture with the. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. First the class is divided into groups of 3 or 4 and the. Molar absorptivity (ε), also known as molar. Wavelength And Absorbance Relationship.
From www.researchgate.net
Relation between absorbance and wavelength for CdO thin films before Wavelength And Absorbance Relationship First the class is divided into groups of 3 or 4 and the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Compare the data for the mixture. Wavelength And Absorbance Relationship.
From public.websites.umich.edu
Chem 125 Experiment II Wavelength And Absorbance Relationship As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Compare the data for the mixture with the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. First the class is divided into groups of 3 or 4 and the. Q 1:what relationship exists between. Wavelength And Absorbance Relationship.
From www.researchgate.net
The relationship between absorbance measured on a spectrophotometer Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because First the class is divided into groups of 3 or 4 and the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? In this experiment students are asked to discover. Wavelength And Absorbance Relationship.
From rheolution.com
Understanding Absorbance at Specific Wavelengths Wavelength And Absorbance Relationship First the class is divided into groups of 3 or 4 and the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Compare the data for the mixture with the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε), also known as molar absorption coefficient. Wavelength And Absorbance Relationship.
From www.animalia-life.club
Wavelength Color Chart Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε), also known. Wavelength And Absorbance Relationship.
From www.researchgate.net
Wavelength absorbance spectrum of the two phases of a biphasic Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because First the class is divided into groups of 3 or 4 and the. Compare the data for the mixture with the. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Molar absorptivity (ε), also known as molar. Wavelength And Absorbance Relationship.
From www.youtube.com
Transmittance and Absorbance and their Relationship with concentration Wavelength And Absorbance Relationship Compare the data for the mixture with the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because As you likely know from other experiences, a particular chemical species absorbs some. Wavelength And Absorbance Relationship.
From www.chegg.com
Solved Absorbance Vs. wavelength Absorbance 0 100 200 500 Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs. Wavelength And Absorbance Relationship.
From www.researchgate.net
Wavelength and absorbance relationship. Download Scientific Diagram Wavelength And Absorbance Relationship Compare the data for the mixture with the. First the class is divided into groups of 3 or 4 and the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific.. Wavelength And Absorbance Relationship.
From www.researchgate.net
Absorbance (cm−1) as a function of wavelength (nm) normalised to 1 mm Wavelength And Absorbance Relationship First the class is divided into groups of 3 or 4 and the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Compare the data for the mixture with the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. As you. Wavelength And Absorbance Relationship.
From www.researchgate.net
Wavelength and absorbance relationship. Download Scientific Diagram Wavelength And Absorbance Relationship In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Compare the data for the mixture with the. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. Q 1:what relationship exists between the wavelength of light absorbed by a solution. Wavelength And Absorbance Relationship.
From www.researchgate.net
Absorbance vs wavelength at 15 min time intervals Download Scientific Wavelength And Absorbance Relationship First the class is divided into groups of 3 or 4 and the. As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Compare the data for the mixture with the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you. Wavelength And Absorbance Relationship.
From www.researchgate.net
The relationship of absorbance with wavelength after 6 h adsorption of Wavelength And Absorbance Relationship As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Compare the data. Wavelength And Absorbance Relationship.
From www.chegg.com
Solved Absorbance vs. wavelength for A Absorbance 400 450 Wavelength And Absorbance Relationship Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you. Wavelength And Absorbance Relationship.
From www.researchgate.net
Graph of wavelength relationship with absorbance in group of treatments Wavelength And Absorbance Relationship As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. First the class. Wavelength And Absorbance Relationship.
From www.chegg.com
Solved Based on the graph of absorbance versus wavelength Wavelength And Absorbance Relationship Compare the data for the mixture with the. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon. Wavelength And Absorbance Relationship.
From www.expii.com
Color and Oxidation State — Overview & Examples Expii Wavelength And Absorbance Relationship In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. First the class is divided into groups of 3 or 4 and the. \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a. Wavelength And Absorbance Relationship.
From www.researchgate.net
Curves of wavelength and absorbance Download Scientific Diagram Wavelength And Absorbance Relationship Compare the data for the mixture with the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. Q 1:what relationship exists between the wavelength of light absorbed by a solution. Wavelength And Absorbance Relationship.
From www.chegg.com
Despite its issues, assume that the graph above is Wavelength And Absorbance Relationship Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because As you likely know from other experiences, a particular chemical species absorbs some wavelengths of radiation and not. Compare the data. Wavelength And Absorbance Relationship.
From www.researchgate.net
Graph of wavelength relationship with absorbance in group of treatments Wavelength And Absorbance Relationship In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Q 1:what relationship exists between the wavelength of light absorbed by a solution of a particular color and the color you see for that solution? Compare the data for the mixture with the. Molar absorptivity (ε), also known as molar absorption coefficient or molar. Wavelength And Absorbance Relationship.
From www.researchgate.net
Sketch map for the relationship between wavelength and absorbance Wavelength And Absorbance Relationship Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a specific. Compare the data for the mixture with the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Q 1:what relationship exists between the wavelength of light absorbed by a solution. Wavelength And Absorbance Relationship.
From www.researchgate.net
The relationship between absorbance (A) and wavelength (λ) Download Wavelength And Absorbance Relationship \[\log \left( \frac{i_{o}}{i} \right)=a=\varepsilon l c\] the absorbance (a) is a unitless number because Compare the data for the mixture with the. In this experiment students are asked to discover the relationship between color, wavelength, absorbance, and concentration. Molar absorptivity (ε), also known as molar absorption coefficient or molar extinction coefficient, measures how strongly a substance absorbs light at a. Wavelength And Absorbance Relationship.