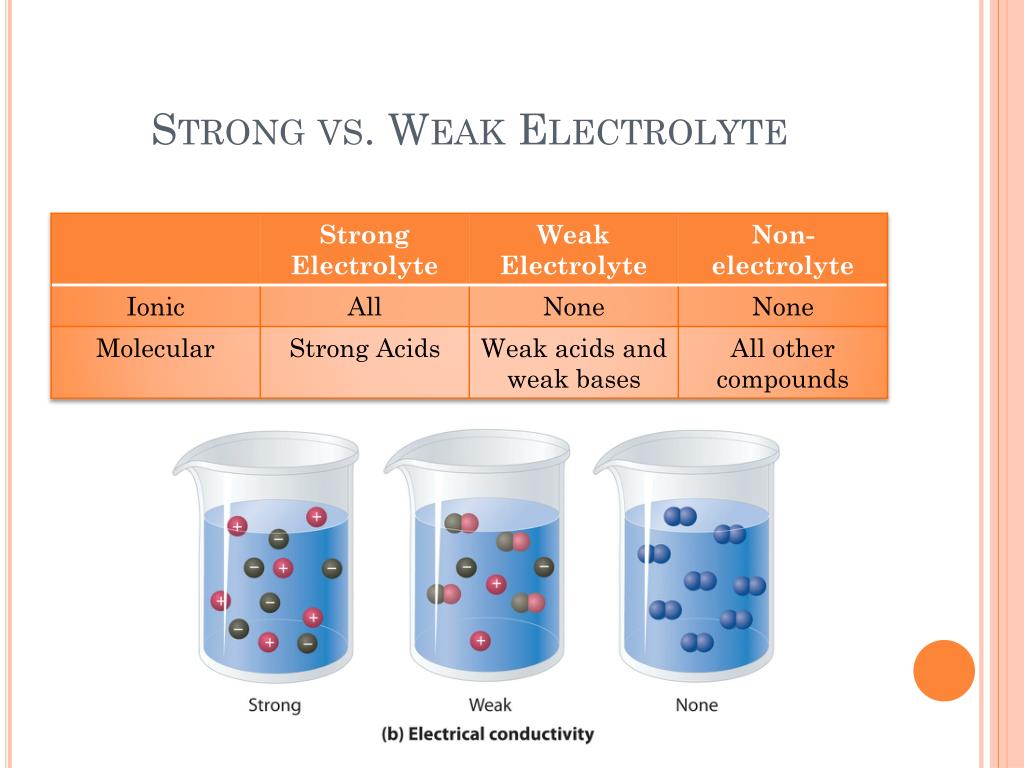
What Is Non Electrolyte Solution . Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte list of typical examples includes: If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Nonelectrolytes tend to be poor. What are examples of nonelectrolytes? A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. Substances, like ionic salts, that dissociate.
from www.slideserve.com
Substances, like ionic salts, that dissociate. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte list of typical examples includes: Nonelectrolytes tend to be poor. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. What are examples of nonelectrolytes?
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry
What Is Non Electrolyte Solution A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. What are examples of nonelectrolytes? Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte list of typical examples includes: Nonelectrolytes tend to be poor. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Substances, like ionic salts, that dissociate. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state.
From www.slideserve.com
PPT Particles in Solution PowerPoint Presentation, free download ID What Is Non Electrolyte Solution Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. What are examples of nonelectrolytes? A nonelectrolyte list of typical examples includes: A nonelectrolyte is a compound that does not conduct an electric current in either. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Solutions Unit PowerPoint Presentation, free download ID1980079 What Is Non Electrolyte Solution An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Nonelectrolytes tend to be poor. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. A nonelectrolyte list of typical examples includes: What are examples of nonelectrolytes?. What Is Non Electrolyte Solution.
From dokumen.tips
(PPTX) Electrolyte and Non Electrolyte Solution DOKUMEN.TIPS What Is Non Electrolyte Solution A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. Substances, like ionic salts, that dissociate. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize. What Is Non Electrolyte Solution.
From courses.lumenlearning.com
Electrolytes Chemistry for Majors What Is Non Electrolyte Solution What are examples of nonelectrolytes? Substances, like ionic salts, that dissociate. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte list of typical examples. What Is Non Electrolyte Solution.
From slideplayer.com
Solutes Electrolytes and Nonelectrolytes ppt download What Is Non Electrolyte Solution If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte list of typical examples includes: Substances, like ionic salts, that dissociate. What are examples of nonelectrolytes? A nonelectrolyte is a substance that. What Is Non Electrolyte Solution.
From www.pinterest.es
To Identify Given Solutions as Electrolyte and Non Electrolyte NEB What Is Non Electrolyte Solution If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Substances, like ionic salts, that dissociate. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a type of substance that does not ionize in either a molten state or. What Is Non Electrolyte Solution.
From killianxinicholson.blogspot.com
Which of the Following Is an Electrolyte Solution What Is Non Electrolyte Solution A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten. What Is Non Electrolyte Solution.
From www.pathwaystochemistry.com
Electrolytes and Nonelectrolytes Pathways to Chemistry What Is Non Electrolyte Solution A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the. What Is Non Electrolyte Solution.
From in.pinterest.com
What is Electrolysis and Electrolyte with examples. Science fair What Is Non Electrolyte Solution A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte list of. What Is Non Electrolyte Solution.
From www.youtube.com
Electrolytes Definition, Examples, & Practice YouTube What Is Non Electrolyte Solution A nonelectrolyte list of typical examples includes: Substances, like ionic salts, that dissociate. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Nonelectrolytes are chemical compounds that, when. What Is Non Electrolyte Solution.
From www.aakash.ac.in
Electrolytes and Nonelectrolytes Types, Differences & Examples AESL What Is Non Electrolyte Solution A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte is a compound that. What Is Non Electrolyte Solution.
From byjus.com
55. The vapour pressure of 2.1 What Is Non Electrolyte Solution What are examples of nonelectrolytes? Substances, like ionic salts, that dissociate. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Nonelectrolytes tend to be poor. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte list of typical examples includes: An electrolyte is any salt or ionizable molecule that, when. What Is Non Electrolyte Solution.
From www.nagwa.com
Question Video Identifying What Type of Electrolyte Sodium Chloride Is What Is Non Electrolyte Solution A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. What are examples of nonelectrolytes? Substances, like ionic salts, that dissociate. A nonelectrolyte is a compound that does not conduct an electric current in either. What Is Non Electrolyte Solution.
From sciencenotes.org
What Are Electrolytes in Chemistry? Strong, Weak, and Non Electrolytes What Is Non Electrolyte Solution A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. If the solution or melt is not electrically conductive, the substance is a. What Is Non Electrolyte Solution.
From www.numerade.com
SOLVEDDistinguish between an electrolyte and a nonelectrolyte. What Is Non Electrolyte Solution An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. Substances, like ionic salts, that dissociate. Nonelectrolytes tend to be poor. A nonelectrolyte list of typical examples includes: Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte is a substance that does. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Solutions Review Pt 2 PowerPoint Presentation, free download ID What Is Non Electrolyte Solution Substances, like ionic salts, that dissociate. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. What are examples of nonelectrolytes? A nonelectrolyte list of typical examples includes: Nonelectrolytes tend to be poor. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID2437162 What Is Non Electrolyte Solution If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte list of typical examples includes: Nonelectrolytes tend to be. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Solution Conductivity PowerPoint Presentation, free download ID What Is Non Electrolyte Solution A nonelectrolyte list of typical examples includes: What are examples of nonelectrolytes? Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes are chemical compounds that, when placed in. What Is Non Electrolyte Solution.
From www.youtube.com
Identifying Strong Electrolytes, Weak Electrolytes, and Nonelectrolytes What Is Non Electrolyte Solution Nonelectrolytes tend to be poor. What are examples of nonelectrolytes? Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. Substances, like ionic salts, that dissociate. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. A nonelectrolyte is a compound that does not conduct an electric current. What Is Non Electrolyte Solution.
From techiescientist.com
Is KOH an Electrolyte? (Detailed Explanation) Techiescientist What Is Non Electrolyte Solution A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes tend to be poor. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. What are examples of nonelectrolytes? An electrolyte is any. What Is Non Electrolyte Solution.
From byjus.com
What is electrolyte and non electrolyte What Is Non Electrolyte Solution Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. Substances, like ionic salts, that dissociate. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. A nonelectrolyte is a type of substance that does not ionize in either. What Is Non Electrolyte Solution.
From www.vecteezy.com
Electrolysis of electrolyte solution, Simple electrolysis process of an What Is Non Electrolyte Solution Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte list of typical examples includes: If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a compound. What Is Non Electrolyte Solution.
From www.w3schools.blog
Strong and weak electrolytes W3schools What Is Non Electrolyte Solution Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. What are examples of nonelectrolytes? A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Warm Up 7.1 Review of Naming & Bonding PowerPoint Presentation What Is Non Electrolyte Solution What are examples of nonelectrolytes? Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte list of typical examples includes: Substances, like ionic salts, that dissociate. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a type of substance that does not ionize in either. What Is Non Electrolyte Solution.
From inspiritvr.com
Electrolytes And NonElectrolytes Study Guide Inspirit What Is Non Electrolyte Solution A nonelectrolyte list of typical examples includes: Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. Nonelectrolytes tend to be poor. If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Substances, like. What Is Non Electrolyte Solution.
From www.slideserve.com
PPT Chapter 4 Aqueous Reactions and Solution Stoichiometry What Is Non Electrolyte Solution A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. What are examples of nonelectrolytes? A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. A nonelectrolyte is a type of substance that does not ionize in either a molten state or in. What Is Non Electrolyte Solution.
From www.youtube.com
7 Electrolyte vs Nonelectrolyte YouTube What Is Non Electrolyte Solution A nonelectrolyte list of typical examples includes: Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability. What Is Non Electrolyte Solution.
From www.animalia-life.club
Electrolyte Chemistry What Is Non Electrolyte Solution Substances, like ionic salts, that dissociate. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in the molten state. A nonelectrolyte list of typical examples includes: Nonelectrolytes are chemical compounds that,. What Is Non Electrolyte Solution.
From hayden-relbrewer.blogspot.com
HaydenRelBrewer What Is Non Electrolyte Solution A nonelectrolyte list of typical examples includes: A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes tend to be poor. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all.. What Is Non Electrolyte Solution.
From general.chemistrysteps.com
General Properties of Solutions Chemistry Steps What Is Non Electrolyte Solution Nonelectrolytes tend to be poor. A nonelectrolyte list of typical examples includes: Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. Substances, like ionic salts, that dissociate. A nonelectrolyte is a type of substance that does not ionize in either. What Is Non Electrolyte Solution.
From www.studypool.com
SOLUTION Electrolyte and non electrolyte solutions Studypool What Is Non Electrolyte Solution If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Nonelectrolytes tend to be poor. Substances, like ionic salts, that dissociate. A nonelectrolyte list of typical examples includes: What are examples of nonelectrolytes? A nonelectrolyte is a type of substance that does not ionize in either a molten state or in solution. Nonelectrolytes are chemical compounds. What Is Non Electrolyte Solution.
From www.shutterstock.com
413 Electrolytic solution Gambar, Foto Stok & Vektor Shutterstock What Is Non Electrolyte Solution What are examples of nonelectrolytes? Substances, like ionic salts, that dissociate. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. If the solution or melt is not electrically conductive, the substance. What Is Non Electrolyte Solution.
From www.vecteezy.com
Electrolysis of electrolyte solution in electrochemistry vector What Is Non Electrolyte Solution If the solution or melt is not electrically conductive, the substance is a nonelectrolyte. Substances, like ionic salts, that dissociate. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. What are examples of nonelectrolytes? An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability. What Is Non Electrolyte Solution.
From ar.inspiredpencil.com
Electrolyte Examples Chemistry What Is Non Electrolyte Solution Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct electricity. A nonelectrolyte list of typical examples includes: A nonelectrolyte is a compound that does not conduct an electric current in either aqueous solution or in. What Is Non Electrolyte Solution.
From byjus.com
A solution of 1.25 g of a non electrolyte in 20 g of water freezes at What Is Non Electrolyte Solution What are examples of nonelectrolytes? Nonelectrolytes tend to be poor. Nonelectrolytes are chemical compounds that, when placed in solution, don’t ionize at all. A nonelectrolyte is a substance that does not exist in an ionic form in aqueous solution. An electrolyte is any salt or ionizable molecule that, when dissolved in solution, will give that solution the ability to conduct. What Is Non Electrolyte Solution.