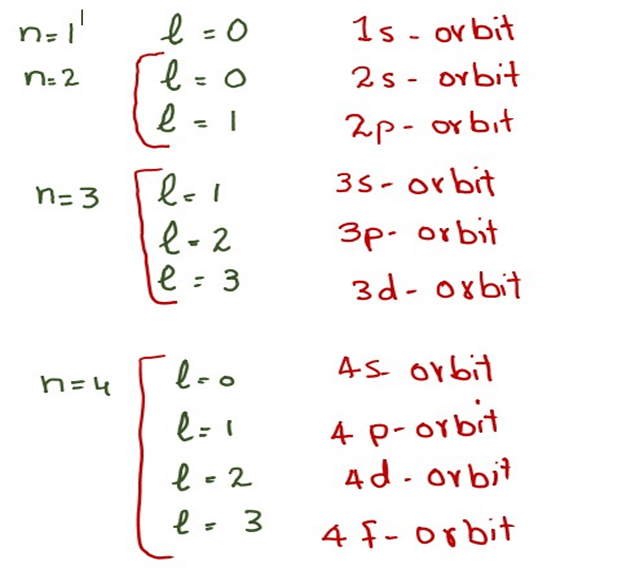
What Is Quantum Numbers In Chemistry . A quantum number is a value that is used when describing the energy levels available to atoms and molecules. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. In atoms, there are a total of four quantum numbers: They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Four have been selected as the electron’s “ quantum numbers.”. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. The quantum numbers are inputs to two key parts of quantum. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Electrons have a few handfuls of properties. Collectively, they all describe the electron configurations.
from eduinput.com
An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. Collectively, they all describe the electron configurations. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement, and energy of the fundamental particle. The quantum numbers are inputs to two key parts of quantum.
Quantum numbersPrinciple, Azimuthal, and Spin
What Is Quantum Numbers In Chemistry The quantum numbers are inputs to two key parts of quantum. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. They represent the position, movement, and energy of the fundamental particle. There are four quantum numbers: Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. Electrons have a few handfuls of properties. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Collectively, they all describe the electron configurations. The quantum numbers are inputs to two key parts of quantum. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Four have been selected as the electron’s “ quantum numbers.”. In atoms, there are a total of four quantum numbers: The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the.
From en.ppt-online.org
Atomic structure and properties. (Chapter 3) online presentation What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. There are four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum. What Is Quantum Numbers In Chemistry.
From www.learninsta.com
Quantum Numbers What Is Quantum Numbers In Chemistry Four have been selected as the electron’s “ quantum numbers.”. In atoms, there are a total of four quantum numbers: There are four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define the state in which a fundamental. What Is Quantum Numbers In Chemistry.
From chem.libretexts.org
8.3 Quantum Numbers for Electrons Chemistry LibreTexts What Is Quantum Numbers In Chemistry Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. The quantum numbers are inputs to two key parts of quantum. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. There are four quantum. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Atomic Structure PowerPoint Presentation, free download ID5937240 What Is Quantum Numbers In Chemistry Four have been selected as the electron’s “ quantum numbers.”. Electrons have a few handfuls of properties. They represent the position, movement, and energy of the fundamental particle. Collectively, they all describe the electron configurations. The quantum numbers are inputs to two key parts of quantum. There are four quantum numbers: An electron in an atom or ion has four. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID6766216 What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. There are four quantum numbers: Four have been selected as the electron’s “ quantum. What Is Quantum Numbers In Chemistry.
From www.madebyteachers.com
Electrons and the 4 Quantum Numbers A Chemistry Worksheet Made By What Is Quantum Numbers In Chemistry Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. Collectively, they all describe the electron configurations. There are four quantum numbers: In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is Quantum Numbers In Chemistry Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons. What Is Quantum Numbers In Chemistry.
From www.priyamstudycentre.com
Quantum Number Orbitals Diagram, Definition, Chart, Shape What Is Quantum Numbers In Chemistry Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. An electron in an atom or ion has four quantum numbers to describe its state and. What Is Quantum Numbers In Chemistry.
From testbook.com
Understand Quantum Numbers Detailed Explanation with Examples What Is Quantum Numbers In Chemistry The quantum numbers are inputs to two key parts of quantum. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. In atoms, there are a total of four quantum numbers: A quantum number is a value that is used when describing the energy levels available to atoms and molecules.. What Is Quantum Numbers In Chemistry.
From www.geeksforgeeks.org
Quantum Numbers Principal, Azimuthal, & Spin What Is Quantum Numbers In Chemistry Four have been selected as the electron’s “ quantum numbers.”. There are four quantum numbers: Collectively, they all describe the electron configurations. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Electrons have a few handfuls of properties. In atoms, there are a total of four quantum numbers: The quantum. What Is Quantum Numbers In Chemistry.
From ar.inspiredpencil.com
Quantum Numbers What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. Collectively, they all describe the electron configurations. Four have been selected as the electron’s “ quantum numbers.”. There are four quantum numbers: Quantum numbers are a set of numbers used to define the. What Is Quantum Numbers In Chemistry.
From chemistryskills.com
Quantum Numbers Chemistry Skills What Is Quantum Numbers In Chemistry They represent the position, movement, and energy of the fundamental particle. The quantum numbers are inputs to two key parts of quantum. In atoms, there are a total of four quantum numbers: Electrons have a few handfuls of properties. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. A. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Unit 2 PowerPoint Presentation, free download ID298336 What Is Quantum Numbers In Chemistry The quantum numbers are inputs to two key parts of quantum. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers are a set of numbers used to define. What Is Quantum Numbers In Chemistry.
From ar.inspiredpencil.com
Quantum Numbers What Is Quantum Numbers In Chemistry Electrons have a few handfuls of properties. There are four quantum numbers: They represent the position, movement, and energy of the fundamental particle. Collectively, they all describe the electron configurations. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron in an atom. What Is Quantum Numbers In Chemistry.
From general.chemistrysteps.com
Quantum Numbers Chemistry Steps What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers: Electrons have a few handfuls of properties. In atoms, there are a total of four quantum numbers: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an. What Is Quantum Numbers In Chemistry.
From www.sliderbase.com
Electron Quantum Numbers Presentation Chemistry What Is Quantum Numbers In Chemistry There are four quantum numbers: The quantum numbers are inputs to two key parts of quantum. Four have been selected as the electron’s “ quantum numbers.”. Collectively, they all describe the electron configurations. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. In atoms, there are a total of. What Is Quantum Numbers In Chemistry.
From www.chemistrylearner.com
Spin Quantum Number Definition, Significance, and Value What Is Quantum Numbers In Chemistry A quantum number is a value that is used when describing the energy levels available to atoms and molecules. They represent the position, movement, and energy of the fundamental particle. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. The quantum numbers are inputs to two key parts of. What Is Quantum Numbers In Chemistry.
From shanimchemistry.blogspot.com
*CHEMISTRY MATRICULATION* QUANTUM NUMBERS What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Collectively, they all describe the electron configurations. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. A quantum number is a value that is used. What Is Quantum Numbers In Chemistry.
From www.chemistrylearner.com
Principal Quantum Number Definition, Determination, & Value What Is Quantum Numbers In Chemistry Electrons have a few handfuls of properties. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. The quantum numbers are parameters that describe the distribution of electrons in the atom,. What Is Quantum Numbers In Chemistry.
From www.youtube.com
UPSC Preparation Quantum numbers YouTube What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. Four have been selected as the electron’s “ quantum numbers.”. The quantum numbers. What Is Quantum Numbers In Chemistry.
From mungfali.com
Quantum Numbers Chart Chemistry What Is Quantum Numbers In Chemistry The quantum numbers are inputs to two key parts of quantum. There are four quantum numbers: In atoms, there are a total of four quantum numbers: Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. Quantum numbers are a set of numbers used to define the state in which. What Is Quantum Numbers In Chemistry.
From eduinput.com
Quantum numbersPrinciple, Azimuthal, and Spin What Is Quantum Numbers In Chemistry The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Electrons have a few handfuls of properties. There are four quantum numbers: Quantum numbers tell us the energy level, the number. What Is Quantum Numbers In Chemistry.
From tuitiontube.com
What is quantum number what do quantum number determine Tuition Tube What Is Quantum Numbers In Chemistry Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. A quantum number is a value that is used when describing the energy levels available to atoms and molecules. Electrons have a few handfuls of properties. The quantum numbers are parameters that describe the distribution. What Is Quantum Numbers In Chemistry.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. They represent the position, movement, and energy of the fundamental particle. In atoms, there are a total of four quantum numbers: A quantum number is a value that is used when describing the. What Is Quantum Numbers In Chemistry.
From general.chemistrysteps.com
s, p, d, f Atomic Orbitals Chemistry Steps What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. In atoms, there are a total of four quantum numbers: Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. The quantum numbers. What Is Quantum Numbers In Chemistry.
From savekhaoyai.blogspot.com
41 quantum number practice worksheet Worksheet Database What Is Quantum Numbers In Chemistry Collectively, they all describe the electron configurations. The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. Electrons have a few handfuls of properties. An electron in an atom or ion. What Is Quantum Numbers In Chemistry.
From ar.inspiredpencil.com
Quantum Numbers What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The quantum numbers are inputs to two key parts of quantum. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. Four have been. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Quantum Numbers PowerPoint Presentation, free download ID3181772 What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The quantum numbers are inputs to two key parts of quantum. Collectively, they all describe the electron configurations. Four have been selected as the electron’s “ quantum numbers.”. They represent the position, movement, and energy of the fundamental particle. Electrons have. What Is Quantum Numbers In Chemistry.
From www.chemistrylearner.com
Spin Quantum Number Definition, Significance, and Value What Is Quantum Numbers In Chemistry In atoms, there are a total of four quantum numbers: Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. They represent the position, movement, and energy of the fundamental particle. A quantum number is a value that is used when describing the energy levels. What Is Quantum Numbers In Chemistry.
From education-portal.com
Spin Quantum Number Definition & Example Video & Lesson Transcript What Is Quantum Numbers In Chemistry An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. The quantum numbers are inputs to two key parts of quantum. Four have been selected as the electron’s “ quantum numbers.”. There are four quantum numbers: Quantum numbers tell us the energy level,. What Is Quantum Numbers In Chemistry.
From study.com
Quantum Numbers on the Periodic Table Definition & Overview Lesson What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. Quantum numbers tell us the energy level, the number and the type of orbitals, and the spin of the electron. In. What Is Quantum Numbers In Chemistry.
From byjus.com
Quantum Numbers (Principal, Azimuthal, and Spin) Definition What Is Quantum Numbers In Chemistry A quantum number is a value that is used when describing the energy levels available to atoms and molecules. The quantum numbers are inputs to two key parts of quantum. Quantum numbers are a set of numbers used to define the state in which a fundamental particle like an atom, ion, nucleus, and electron resides. An electron in an atom. What Is Quantum Numbers In Chemistry.
From www.slideserve.com
PPT Electron Configurations & Quantum Numbers PowerPoint Presentation What Is Quantum Numbers In Chemistry The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave equation for the hydrogen atom. Four have been selected as the electron’s “ quantum numbers.”. They represent the position,. What Is Quantum Numbers In Chemistry.
From www.pinterest.ph
Quantum Number definition A number assigned to each electron orbit of What Is Quantum Numbers In Chemistry The quantum numbers are inputs to two key parts of quantum. Collectively, they all describe the electron configurations. The principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (m l), and the. An electron in an atom or ion has four quantum numbers to describe its state and yield solutions to the schrödinger wave. What Is Quantum Numbers In Chemistry.
From socratic.org
What are the four quantum numbers in chemistry? Socratic What Is Quantum Numbers In Chemistry In atoms, there are a total of four quantum numbers: The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. There are four quantum numbers: Four have been selected as the electron’s “ quantum numbers.”. Quantum numbers tell us the energy level, the number and the type of orbitals, and the. What Is Quantum Numbers In Chemistry.