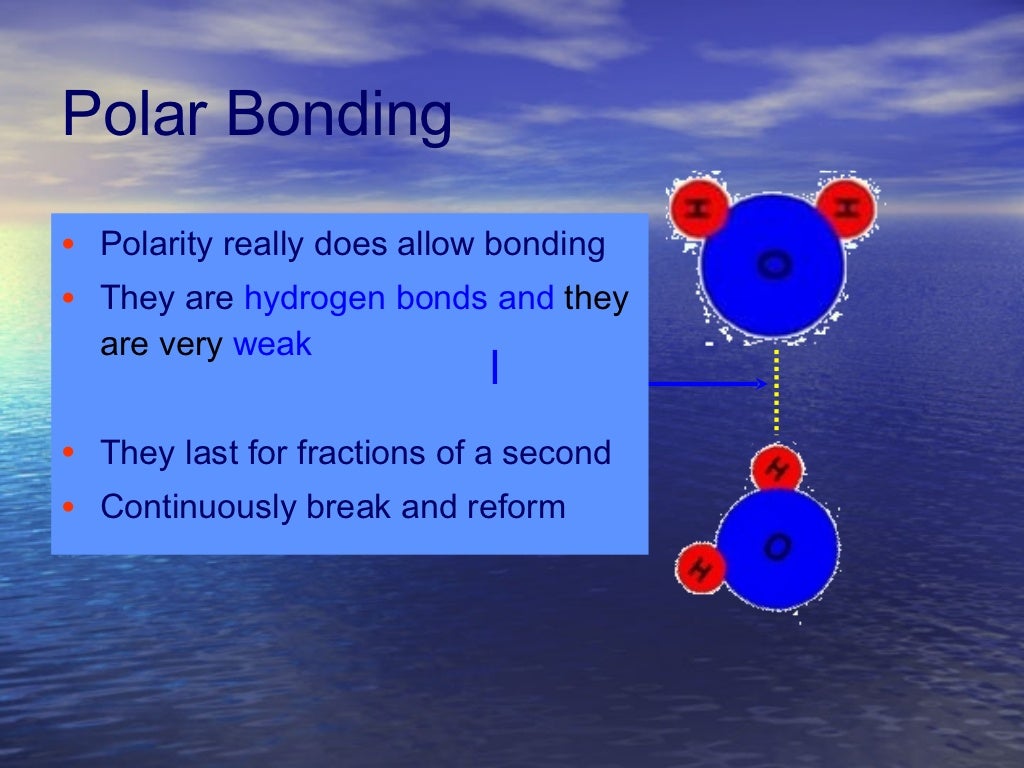
Water Polarity Definition . The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The hydrogen and oxygen within water molecules (h 2 o). learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. water (h 2 o) is polar because of the bent shape of the molecule. This is an example of polar covalent chemical bonding. Explore how water's unique states and heat capacity are. When a polar substance is put in water, the positive ends of its molecules are. One of water’s important properties is that it is composed of polar molecules: water’s polarity allows it to dissolve other polar substances very easily. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar.
from www.slideshare.net
learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The hydrogen and oxygen within water molecules (h 2 o). polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. When a polar substance is put in water, the positive ends of its molecules are. One of water’s important properties is that it is composed of polar molecules: Explore how water's unique states and heat capacity are. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. water’s polarity allows it to dissolve other polar substances very easily.
Water's Polarity
Water Polarity Definition water (h 2 o) is polar because of the bent shape of the molecule. The hydrogen and oxygen within water molecules (h 2 o). learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. water’s polarity allows it to dissolve other polar substances very easily. One of water’s important properties is that it is composed of polar molecules: water (h 2 o) is polar because of the bent shape of the molecule. Explore how water's unique states and heat capacity are. When a polar substance is put in water, the positive ends of its molecules are. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example of polar covalent chemical bonding. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms.
From www.vrogue.co
What Are Polarity Definition Types And Importance Che vrogue.co Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. Explore how water's unique states and heat capacity are. The hydrogen and oxygen within water molecules (h 2 o). The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other. Water Polarity Definition.
From chemistrytalk.org
Polarity of Water Why is Water Polar? ChemTalk Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. This is an example of polar covalent chemical bonding. The shape means most of the negative charge from the oxygen on. Water Polarity Definition.
From www.slideserve.com
PPT Ch 3 The Polarity of Water and Its Properties PowerPoint Water Polarity Definition This is an example of polar covalent chemical bonding. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. When a polar substance is put in water, the positive ends of its molecules are. learn how. Water Polarity Definition.
From www.slideserve.com
PPT 22 Properties of water PowerPoint Presentation, free download Water Polarity Definition This is an example of polar covalent chemical bonding. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. One of water’s important properties is that it is composed of polar molecules: water’s polarity allows it to dissolve other polar substances very easily. learn how water molecules have partial charges due to their polar. Water Polarity Definition.
From www.toppr.com
How to Explain Polarity, How to Determine Polarity Water Polarity Definition learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. water’s polarity allows it to dissolve other polar substances very easily. When a polar substance is put in water, the positive ends of its molecules are. polarity refers to the distribution of electric charges in a molecule, which creates. Water Polarity Definition.
From www.thoughtco.com
Polar Molecule Definition and Examples Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. This is an example of polar covalent chemical bonding. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. Explore how water's unique states and. Water Polarity Definition.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. water (h 2 o) is polar because of the bent shape of the molecule. This is an example of polar covalent chemical bonding. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. water’s. Water Polarity Definition.
From socratic.org
What kinds of molecules are polar? + Example Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. learn how water is polar due to its bent geometry and electronegativity difference. Water Polarity Definition.
From www.slideserve.com
PPT Marine Biology Lesson 3 PowerPoint Presentation, free download Water Polarity Definition This is an example of polar covalent chemical bonding. One of water’s important properties is that it is composed of polar molecules: polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water molecules have partial charges due to their polar covalent bonds and how they. Water Polarity Definition.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. When a polar substance is put in water, the positive ends of its molecules are. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms. Water Polarity Definition.
From www.slideserve.com
PPT Water as a Polar Molecule PowerPoint Presentation, free download Water Polarity Definition water (h 2 o) is polar because of the bent shape of the molecule. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. One of water’s important properties is that it is composed of polar molecules: The hydrogen and oxygen within water molecules (h 2 o). This is. Water Polarity Definition.
From www.youtube.com
Polarity of Water Molecule Explained YouTube Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. water (h 2 o) is polar because of the bent shape of the molecule. This is an example of polar covalent chemical bonding. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. learn. Water Polarity Definition.
From www.slideserve.com
PPT Water and life PowerPoint Presentation, free download ID1459288 Water Polarity Definition polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. water (h 2 o) is polar because of the bent shape of the molecule. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. Explore how water's unique states. Water Polarity Definition.
From www.slideserve.com
PPT Polarity of water PowerPoint Presentation, free download ID2610682 Water Polarity Definition learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. When a polar substance is put in water, the positive ends of its molecules are. The hydrogen and oxygen within water molecules (h 2 o). learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. learn. Water Polarity Definition.
From www.amoebasisters.com
Water the Polar Molecule SCIENCE WITH THE AMOEBA SISTERS Water Polarity Definition polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. This is an example of polar covalent chemical bonding. One of water’s important properties is that it is composed of polar molecules: learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. The shape means. Water Polarity Definition.
From humanbiology.pressbooks.tru.ca
3.11 Water and Life Human Biology Water Polarity Definition Explore how water's unique states and heat capacity are. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. water’s polarity allows. Water Polarity Definition.
From www.slideserve.com
PPT Water Chemistry PowerPoint Presentation, free download ID6898540 Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. Explore how water's unique states and heat capacity are. polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water molecules have partial charges due to their polar covalent bonds and how they attract. Water Polarity Definition.
From webmis.highland.cc.il.us
Aqueous Solutions Water Polarity Definition polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar.. Water Polarity Definition.
From www.aakash.ac.in
Polarity in Chemistry Definition, Types and Importance of Polarity AESL Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. water (h 2 o) is polar because of the bent shape of the molecule. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms. Water Polarity Definition.
From study.com
Polar Molecule Definition & Examples Video & Lesson Transcript Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. One of water’s important properties is that it is composed of polar molecules: water’s polarity allows it to dissolve other polar substances very easily. When a polar substance is put in water, the positive ends of its molecules are.. Water Polarity Definition.
From www.slideserve.com
PPT Biology Chapter 6 PowerPoint Presentation, free download ID2514063 Water Polarity Definition learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on. Water Polarity Definition.
From www.slideshare.net
Water's Polarity Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. Explore how water's unique states and heat capacity are. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of. Water Polarity Definition.
From nl.pinterest.com
Why Is Water a Polar Molecule? Hydrogen Atom, Hydrogen Bond, Ionic Water Polarity Definition The hydrogen and oxygen within water molecules (h 2 o). learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. One of water’s important properties is that it is composed of polar molecules: Explore how water's unique states and heat capacity are. When a polar substance is put in water, the positive ends of its molecules. Water Polarity Definition.
From www.thoughtco.com
Definition and Examples of a Polar Bond Water Polarity Definition polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. This is an example of polar covalent chemical bonding. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side. Water Polarity Definition.
From sciencetrends.com
Polar Molecule Definition And Examples Science Trends Water Polarity Definition learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. The hydrogen and oxygen within water molecules (h 2 o). water (h 2 o) is polar because of the bent shape of the molecule. Explore how water's unique states and heat capacity are. water’s polarity allows it to dissolve other polar substances very easily.. Water Polarity Definition.
From www.slideserve.com
PPT Biology SOL Review PowerPoint Presentation, free download ID Water Polarity Definition When a polar substance is put in water, the positive ends of its molecules are. water’s polarity allows it to dissolve other polar substances very easily. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule.. Water Polarity Definition.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. When a polar substance is put in water, the positive ends of its molecules are. polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. One of water’s important. Water Polarity Definition.
From www.slideshare.net
Water's Polarity Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. Explore how water's unique states and heat capacity are. The hydrogen and oxygen within water molecules (h 2 o). This is an example of polar covalent chemical. Water Polarity Definition.
From biologydictionary.net
Polar Molecule Definition and Examples Biology Dictionary Water Polarity Definition learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. One of water’s important properties is that it is composed of polar molecules: polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. The shape means most of the. Water Polarity Definition.
From www.slideshare.net
Water's Polarity Water Polarity Definition learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. Explore how water's unique states and heat capacity are. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. This is an example. Water Polarity Definition.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Water Polarity Definition The hydrogen and oxygen within water molecules (h 2 o). One of water’s important properties is that it is composed of polar molecules: polarity refers to the distribution of electric charges in a molecule, which creates a difference in electric potential across the. Explore how water's unique states and heat capacity are. The shape means most of the negative. Water Polarity Definition.
From www.slideserve.com
PPT INTERACTIONS OF MATTER PowerPoint Presentation, free download Water Polarity Definition learn how water is polar due to its bent geometry and electronegativity difference between hydrogen and oxygen atoms. learn how water molecules have partial charges due to their polar covalent bonds and how they attract other polar. Explore how water's unique states and heat capacity are. The shape means most of the negative charge from the oxygen on. Water Polarity Definition.
From chemistrytalk.org
Polarity of Water Why is Water Polar? ChemTalk Water Polarity Definition water’s polarity allows it to dissolve other polar substances very easily. The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. The hydrogen and oxygen within water molecules (h 2 o). One of water’s important properties. Water Polarity Definition.
From www.youtube.com
Polar Molecules Tutorial How to determine polarity in a molecule YouTube Water Polarity Definition The shape means most of the negative charge from the oxygen on one side of the molecule and the positive charge of the hydrogen atoms is on the other side of the molecule. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion, adhesion, and ph. water’s polarity allows it to dissolve other polar substances very easily. . Water Polarity Definition.
From www.slideshare.net
Water's Polarity Water Polarity Definition One of water’s important properties is that it is composed of polar molecules: water (h 2 o) is polar because of the bent shape of the molecule. Explore how water's unique states and heat capacity are. water’s polarity allows it to dissolve other polar substances very easily. learn how water's polarity leads to hydrogen bonding, dissolution, cohesion,. Water Polarity Definition.