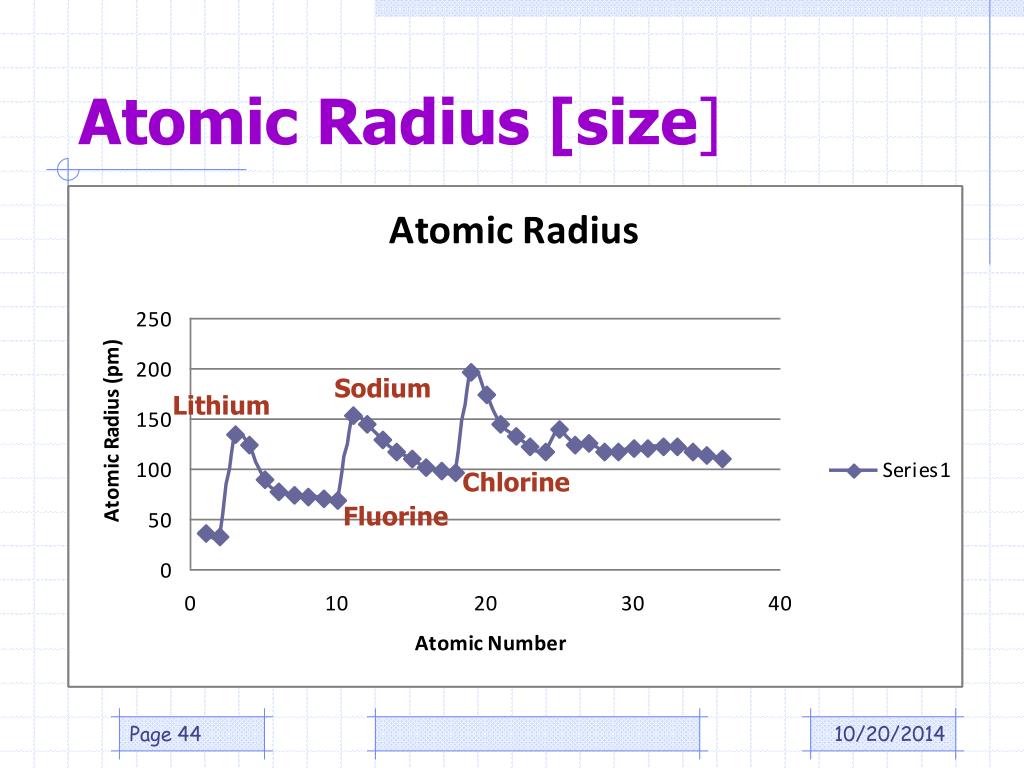
Atomic Radius Size Chlorine . 119 rows it is often denoted by a0 and is approximately 53 pm. Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). 119 rows chlorine (cl) 175 pm We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Trends in atomic radius in the periodic table. As the atomic number increases, the atomic radius decreases. Some are bound by covalent bonds in molecules, some are attracted to each other. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. The graph shows how atomic radius varies across period 3:
from www.slideserve.com
We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Some are bound by covalent bonds in molecules, some are attracted to each other. As the atomic number increases, the atomic radius decreases. 119 rows chlorine (cl) 175 pm Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). 119 rows it is often denoted by a0 and is approximately 53 pm. The graph shows how atomic radius varies across period 3: Trends in atomic radius in the periodic table. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way.
PPT Periodic Table PowerPoint Presentation, free download ID5631786
Atomic Radius Size Chlorine As the atomic number increases, the atomic radius decreases. Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). As the atomic number increases, the atomic radius decreases. Some are bound by covalent bonds in molecules, some are attracted to each other. The graph shows how atomic radius varies across period 3: 119 rows it is often denoted by a0 and is approximately 53 pm. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. 119 rows chlorine (cl) 175 pm Trends in atomic radius in the periodic table.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID9646789 Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). As the atomic number increases, the atomic radius decreases. Some are bound by covalent. Atomic Radius Size Chlorine.
From chem.libretexts.org
9.9 Periodic Trends Atomic Size, Ionization Energy, and Metallic Atomic Radius Size Chlorine Some are bound by covalent bonds in molecules, some are attracted to each other. Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent. Atomic Radius Size Chlorine.
From stock.adobe.com
Chlorine big on periodic Table of the Elements with atomic number Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm Some are bound by covalent bonds in molecules, some are attracted to each other. Trends in atomic radius in the periodic table. As the atomic number increases, the atomic radius decreases. The graph shows how atomic radius varies across period 3: However, this idea is complicated by the fact that not all atoms are. Atomic Radius Size Chlorine.
From avopix.com
Calculate Atomic Radius using diatomic molecules Royalty Free Stock Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in. Atomic Radius Size Chlorine.
From www.doubtnut.com
(a) Give the schematic atomic structures of chlorine atom and chloride Atomic Radius Size Chlorine As the atomic number increases, the atomic radius decreases. Trends in atomic radius in the periodic table. 119 rows chlorine (cl) 175 pm The graph shows how atomic radius varies across period 3: However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Some are bound by covalent bonds in. Atomic Radius Size Chlorine.
From www.youtube.com
Atomic Structure (Bohr Model) for Chlorine (Cl) YouTube Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Some are bound by covalent bonds in molecules, some are attracted to each other. The. Atomic Radius Size Chlorine.
From sciencenotes.org
Chlorine Facts Atomic Radius Size Chlorine Trends in atomic radius in the periodic table. The graph shows how atomic radius varies across period 3: Some are bound by covalent bonds in molecules, some are attracted to each other. 119 rows chlorine (cl) 175 pm Hence, the values of atomic radii given here in picometers can be converted. 119 rows it is often denoted by a0 and. Atomic Radius Size Chlorine.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Atomic Radius Size Chlorine 119 rows it is often denoted by a0 and is approximately 53 pm. Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond. Atomic Radius Size Chlorine.
From www.dreamstime.com
An Atom of Chlorine Diagram Stock Vector Illustration of structure Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond. Atomic Radius Size Chlorine.
From bradleyrahman.z13.web.core.windows.net
Chart Of Atomic Radius Of Elements Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). 119 rows chlorine (cl) 175 pm As the atomic number increases, the atomic radius. Atomic Radius Size Chlorine.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Atomic Radius Size Chlorine However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99. Atomic Radius Size Chlorine.
From www.periodictableprintable.com
Periodic Table Decreasing Atomic Radius 2024 Periodic Table Printable Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Trends in atomic radius in the periodic table. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. The. Atomic Radius Size Chlorine.
From www.shutterstock.com
Types Atomic Radius Chemical Element Nitrogen เวกเตอร์สต็อก (ปลอดค่า Atomic Radius Size Chlorine As the atomic number increases, the atomic radius decreases. 119 rows it is often denoted by a0 and is approximately 53 pm. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Some are bound by covalent bonds in molecules, some are attracted to each. Atomic Radius Size Chlorine.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Atomic Radius Size Chlorine Hence, the values of atomic radii given here in picometers can be converted. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). 119. Atomic Radius Size Chlorine.
From www.shutterstock.com
Types Atomic Radius Chemical Element Atomic Stock Vector (Royalty Free Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm Trends in atomic radius in the periodic table. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. The graph shows how atomic radius varies across period 3: We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius. Atomic Radius Size Chlorine.
From courses.lumenlearning.com
Periodic Variations in Element Properties CHEM 1305 General Atomic Radius Size Chlorine However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. 119 rows it is often denoted by a0 and is approximately 53 pm. 119 rows chlorine (cl) 175 pm Trends in atomic radius in the periodic table. As the atomic number increases, the atomic radius decreases. Hence, the values of. Atomic Radius Size Chlorine.
From stock.adobe.com
Chlorine atomic structure has atomic number, atomic mass, electron Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in. Atomic Radius Size Chlorine.
From www.alamy.com
3d render of atom structure of chlorine isolated over white background Atomic Radius Size Chlorine The graph shows how atomic radius varies across period 3: However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. 119 rows it is often denoted by a0 and is approximately 53 pm. Hence, the values of atomic radii given here in picometers can be converted. 119 rows chlorine (cl). Atomic Radius Size Chlorine.
From www.embibe.com
Draw the atomic structure of the Chlorine atom and chlorine ion Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm As the atomic number increases, the atomic radius decreases. Some are bound by covalent bonds in molecules, some are attracted to each other. Hence, the values of atomic radii given here in picometers can be converted. The graph shows how atomic radius varies across period 3: We assign half of this distance to each. Atomic Radius Size Chlorine.
From favpng.com
Atom Bohr Model Electron Configuration Chlorine, PNG, 1000x1000px, Atom Atomic Radius Size Chlorine However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. As the atomic number increases, the atomic radius decreases. Hence, the values of atomic radii given here in picometers can be converted. 119 rows it is often denoted by a0 and is approximately 53 pm. Some are bound by covalent. Atomic Radius Size Chlorine.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Atomic Radius Size Chlorine Some are bound by covalent bonds in molecules, some are attracted to each other. 119 rows it is often denoted by a0 and is approximately 53 pm. As the atomic number increases, the atomic radius decreases. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the. Atomic Radius Size Chlorine.
From mindsstorm.weebly.com
Atomic radius chart mindsstorm Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Some are bound by covalent bonds in molecules, some are attracted to each other. 119 rows chlorine (cl) 175 pm Hence, the values of atomic radii given here in picometers can be converted. Trends in. Atomic Radius Size Chlorine.
From science4fun.info
Chlorine Element (Properties, Uses, and Facts) Science4Fun Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. 119 rows chlorine (cl) 175 pm The graph shows how atomic radius varies across period 3: Trends in atomic radius in the periodic table. We assign half of this distance to each chlorine atom, giving. Atomic Radius Size Chlorine.
From www.chem.fsu.edu
Electron Configurations Atomic Radius Size Chlorine We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). As the atomic number increases, the atomic radius decreases. We assign half of this. Atomic Radius Size Chlorine.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Atomic Radius Size Chlorine Some are bound by covalent bonds in molecules, some are attracted to each other. The graph shows how atomic radius varies across period 3: However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Trends in atomic radius in the periodic table. 119 rows chlorine (cl) 175 pm 119 rows. Atomic Radius Size Chlorine.
From www.flinnsci.ca
Atomic Sizes and Radii Charts for Chemistry Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm Hence, the values of atomic radii given here in picometers can be converted. As the atomic number increases, the atomic radius decreases. 119 rows it is often denoted by a0 and is approximately 53 pm. Some are bound by covalent bonds in molecules, some are attracted to each other. Trends in atomic radius in. Atomic Radius Size Chlorine.
From www.alamy.com
chlorine chemical element isotopes atomic structure illustration Atomic Radius Size Chlorine As the atomic number increases, the atomic radius decreases. The graph shows how atomic radius varies across period 3: We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius. Atomic Radius Size Chlorine.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Atomic Radius Size Chlorine As the atomic number increases, the atomic radius decreases. Some are bound by covalent bonds in molecules, some are attracted to each other. 119 rows it is often denoted by a0 and is approximately 53 pm. Hence, the values of atomic radii given here in picometers can be converted. 119 rows chlorine (cl) 175 pm We assign half of this. Atomic Radius Size Chlorine.
From payscalechart.z28.web.core.windows.net
atomic scale chart Table periodic chemistry elements chemicool Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm Some are bound by covalent bonds in molecules, some are attracted to each other. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Trends in atomic radius in the periodic table. Hence, the values of atomic radii given. Atomic Radius Size Chlorine.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Atomic Radius Size Chlorine Hence, the values of atomic radii given here in picometers can be converted. As the atomic number increases, the atomic radius decreases. 119 rows it is often denoted by a0 and is approximately 53 pm. The graph shows how atomic radius varies across period 3: However, this idea is complicated by the fact that not all atoms are normally bound. Atomic Radius Size Chlorine.
From www.webelements.com
Elements Periodic Table » Chlorine » properties of free atoms Atomic Radius Size Chlorine 119 rows chlorine (cl) 175 pm We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). However, this idea is complicated by the fact. Atomic Radius Size Chlorine.
From chemistry.stackexchange.com
What is the atomic radius of chlorine? Chemistry Stack Exchange Atomic Radius Size Chlorine However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. As the atomic number increases, the atomic radius decreases. 119 rows chlorine (cl) 175 pm Some are bound by covalent bonds in molecules, some are attracted to each other. We assign half of this distance to each chlorine atom, giving. Atomic Radius Size Chlorine.
From www.slideserve.com
PPT Periodic Table PowerPoint Presentation, free download ID5631786 Atomic Radius Size Chlorine Trends in atomic radius in the periodic table. 119 rows it is often denoted by a0 and is approximately 53 pm. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Some are bound by covalent bonds in molecules, some are attracted to each other. As the atomic number increases,. Atomic Radius Size Chlorine.
From www.alamy.com
Chlorine electron configuration. Illustration of the atomic structure Atomic Radius Size Chlorine However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. 119 rows chlorine (cl) 175 pm We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (r cov), which is half the distance between the. Some are bound by covalent bonds in molecules, some. Atomic Radius Size Chlorine.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Atomic Radius Size Chlorine Trends in atomic radius in the periodic table. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a covalent bond in the same molecule, of 99 pm or 0.99 å (figure \(\pageindex{2a}\)). We assign half of this distance. Atomic Radius Size Chlorine.