Which Substances Dissolve In Water Check All Of The Boxes That Apply . Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Its ionic nature allows it to dissolve easily in water. Nonpolar molecules such as those. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonelectrolytes are substances that do not produce ions when. Which substances are most likely to undergo dissolution in water?
from www.dreamstime.com
In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Which substances are most likely to undergo dissolution in water? Nonpolar molecules such as those. Substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. Its ionic nature allows it to dissolve easily in water.
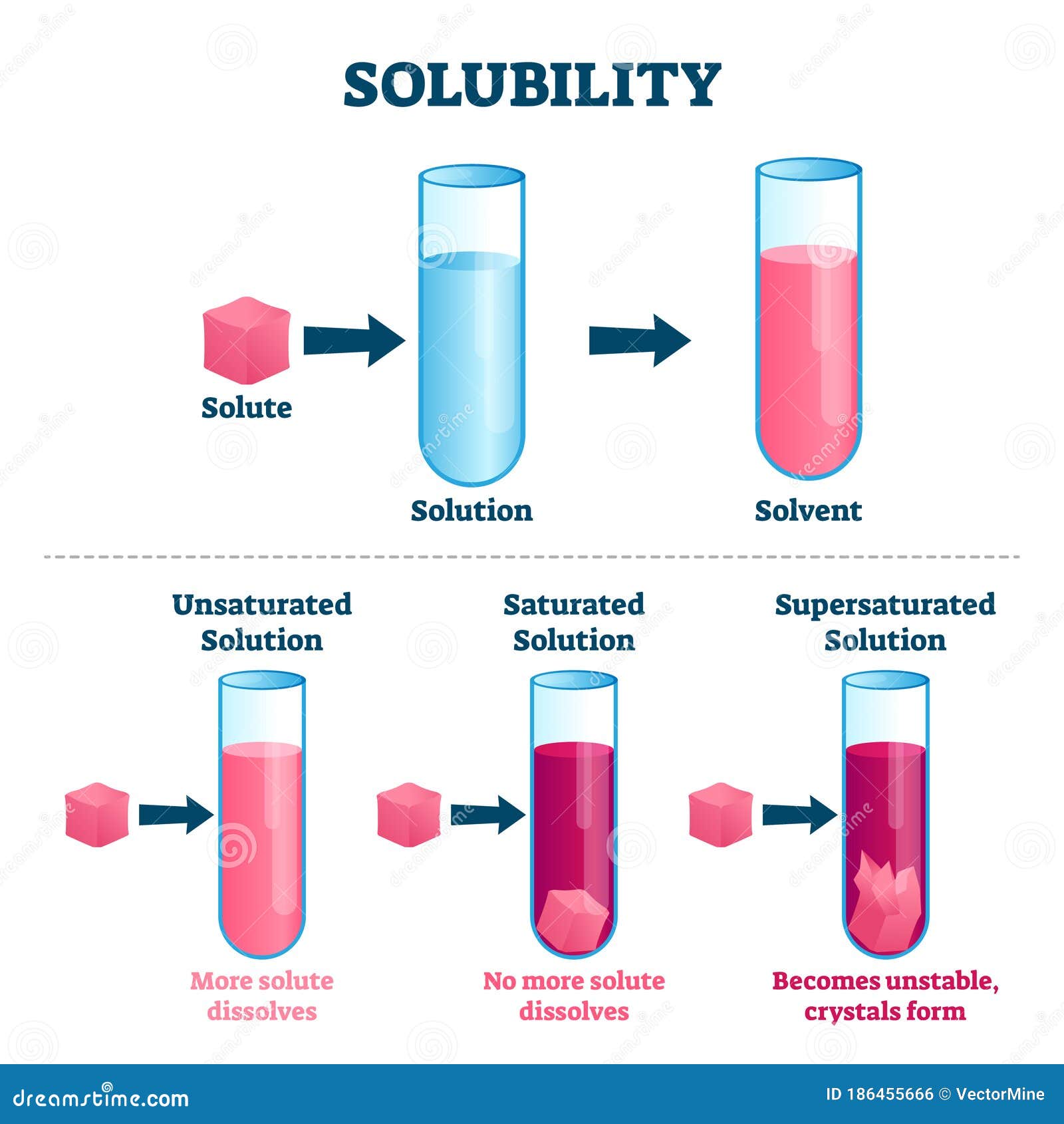
Solubility Vector Illustration. Labeled Solute, Solvent and Solution
Which Substances Dissolve In Water Check All Of The Boxes That Apply Its ionic nature allows it to dissolve easily in water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonpolar molecules such as those. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Which substances are most likely to undergo dissolution in water? Nonelectrolytes are substances that do not produce ions when. Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves many ionic compounds and polar molecules. Its ionic nature allows it to dissolve easily in water. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻).
From www.science-sparks.com
Which Solids Dissolve In Water Cool Science for Kids Which Substances Dissolve In Water Check All Of The Boxes That Apply In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Its ionic nature allows it to dissolve easily in water. Which substances are most likely to undergo dissolution in water? In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Water typically dissolves many. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.vrogue.co
What Is Solubility Definition Solubility Product Fact vrogue.co Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Nonelectrolytes are substances that do not produce ions when. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonpolar molecules such as those. In order for a nonpolar. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.chegg.com
Solved Part C he following substances dissolve when added to Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Water typically dissolves many ionic compounds and polar molecules. Which substances are most likely to undergo dissolution in water? In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Its ionic nature allows it to dissolve easily in water.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From sierraukung.blogspot.com
Discussion Of Absorbing Carbon Dioxide In Water Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Its ionic nature allows it to dissolve easily in water. Substances that dissolve in water to yield. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.slideserve.com
PPT To understand the process of dissolving To learn why certain Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Nonpolar molecules such as those. Substances that dissolve in water to yield ions are called electrolytes. Nonelectrolytes are substances that do not produce ions when. Study with quizlet and. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.chegg.com
Solved When the following substances dissolve in water, what Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Nonelectrolytes are. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER Which Substances Dissolve In Water Check All Of The Boxes That Apply In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Substances that dissolve in water to yield ions. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From byjus.com
Which among the following things do not dissolve in water? Which Substances Dissolve In Water Check All Of The Boxes That Apply Which substances are most likely to undergo dissolution in water? Nonpolar molecules such as those. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Nonelectrolytes are substances that do not produce ions when.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From bceweb.org
Solubility Of Compounds In Water Chart A Visual Reference of Charts Which Substances Dissolve In Water Check All Of The Boxes That Apply Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Its ionic nature allows it to dissolve easily in water. Nonpolar molecules such as those. Which substances. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.youtube.com
Which Solids dissolve in water Science Experiments for Kids Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Substances that dissolve in water to yield ions are called electrolytes. In order for a nonpolar molecule to dissolve in water, it would need to break. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.numerade.com
SOLVED Five different substances are given to you to be dissolved in Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves many ionic compounds and polar molecules. Nonelectrolytes are substances that do not produce ions when.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From brainly.in
the substance that will not dissolve in water Brainly.in Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In order for a nonpolar molecule to dissolve in water, it would need to break up some. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.chegg.com
Solved Which molecular substances dissolve in water? Why? Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Water typically dissolves many ionic compounds and. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From gracelimlf.blogspot.com
LIM LAI FONG (D20102043844) Science Year 3 Which Substances Dissolve In Water Check All Of The Boxes That Apply Water typically dissolves many ionic compounds and polar molecules. Its ionic nature allows it to dissolve easily in water. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From slidetodoc.com
HOW AND WHY DO SUBSTANCES DISSOLVE IN WATER Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonelectrolytes are substances that do not produce ions when. In order for a nonpolar molecule to dissolve in water, it would need to break up some. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.alamy.com
Dissolving science experiment with sugar dissolve in water illustration Which Substances Dissolve In Water Check All Of The Boxes That Apply Substances that dissolve in water to yield ions are called electrolytes. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Which substances are most likely to undergo dissolution in water? Its ionic nature allows it to dissolve easily in water. Water typically dissolves many ionic compounds and. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.chegg.com
Solved 2. The following compounds dissolve readily in water. Which Substances Dissolve In Water Check All Of The Boxes That Apply Water typically dissolves many ionic compounds and polar molecules. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Which substances are most likely. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From brainly.com
Which of the following substances most likely dissolve in water? Check Which Substances Dissolve In Water Check All Of The Boxes That Apply Which substances are most likely to undergo dissolution in water? Substances that dissolve in water to yield ions are called electrolytes. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Its ionic nature allows it to dissolve easily in water. Water typically dissolves many ionic compounds and polar molecules. In order for a nonpolar molecule to dissolve. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.slideserve.com
PPT Chapter 8 SOLUTIONS PowerPoint Presentation, free download ID Which Substances Dissolve In Water Check All Of The Boxes That Apply In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Nonelectrolytes are substances that do not produce ions when. Which substances are most likely to undergo dissolution in water? Substances that dissolve in water to yield ions are called electrolytes. Water typically dissolves many ionic compounds. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.chegg.com
Solved Five different substances are given to you to be Which Substances Dissolve In Water Check All Of The Boxes That Apply Water typically dissolves many ionic compounds and polar molecules. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Nonpolar molecules such as those. Its ionic nature allows it to dissolve easily in water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonelectrolytes are. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.dreamstime.com
Solubility Vector Illustration. Labeled Solute, Solvent and Solution Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Nonpolar molecules such as those. Water typically dissolves many ionic compounds and polar molecules. Its ionic nature allows it to dissolve easily in water. Substances that dissolve in water to yield ions are called electrolytes. Study with quizlet and memorize flashcards. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.slideserve.com
PPT To understand the process of dissolving To learn why certain Which Substances Dissolve In Water Check All Of The Boxes That Apply Its ionic nature allows it to dissolve easily in water. Water typically dissolves many ionic compounds and polar molecules. Substances that dissolve in water to yield ions are called electrolytes. Which substances are most likely to undergo dissolution in water? Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Study. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From ar.inspiredpencil.com
Solubility In Water Which Substances Dissolve In Water Check All Of The Boxes That Apply Which substances are most likely to undergo dissolution in water? Nonelectrolytes are substances that do not produce ions when. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Its ionic nature allows it to dissolve easily in water. Nonpolar molecules such as those. In water,. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.twinkl.com.cn
What is Dissolving? Answered Twinkl Teaching Wiki Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Nonelectrolytes are substances that do not produce ions when. Which substances are most likely to undergo dissolution in water? Nonpolar molecules such as those. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In order. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.cbsetuts.com
Which of the following substances are insoluble in water? CBSE Tuts Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonpolar molecules such as those. Water typically dissolves many ionic compounds and polar molecules. Which substances are most likely to undergo dissolution in water? Study with quizlet and memorize flashcards containing terms like which compounds will most likely. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID2869731 Which Substances Dissolve In Water Check All Of The Boxes That Apply Which substances are most likely to undergo dissolution in water? Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Its ionic nature allows it to dissolve easily in water. Substances that dissolve in water to yield ions are called electrolytes. In water, it dissociates into sodium ions. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.youtube.com
Which substances dissolve in water? YouTube Which Substances Dissolve In Water Check All Of The Boxes That Apply Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Nonelectrolytes are substances that do not produce ions when. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.numerade.com
SOLVED The following substances dissolve when added to water Classify Which Substances Dissolve In Water Check All Of The Boxes That Apply Its ionic nature allows it to dissolve easily in water. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.coursehero.com
[Solved] Five different substances are given to you to be dissolved in Which Substances Dissolve In Water Check All Of The Boxes That Apply Substances that dissolve in water to yield ions are called electrolytes. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. Which substances are most likely to undergo dissolution in water? In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From brainly.com
Question Use the table to compare the solubilities of substances Which Substances Dissolve In Water Check All Of The Boxes That Apply Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From study.com
Compound Solubility in Water Overview & Examples Lesson Which Substances Dissolve In Water Check All Of The Boxes That Apply Nonpolar molecules such as those. Nonelectrolytes are substances that do not produce ions when. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Which substances are most likely to undergo dissolution in water? Substances that dissolve in water to yield ions are called electrolytes. In order for a nonpolar molecule to dissolve in water, it would need. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From oneclass.com
OneClass how many grams of each of the following substances will Which Substances Dissolve In Water Check All Of The Boxes That Apply Which substances are most likely to undergo dissolution in water? Its ionic nature allows it to dissolve easily in water. In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Water typically dissolves many ionic compounds and polar molecules. Study with quizlet and memorize flashcards containing terms like which compounds will most likely dissociate when dissolved in water?.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.twinkl.co.cr
What is Dissolving? Answered Twinkl Teaching Wiki Which Substances Dissolve In Water Check All Of The Boxes That Apply In water, it dissociates into sodium ions (na⁺) and nitrate ions (no₃⁻). Water typically dissolves many ionic compounds and polar molecules. Substances that dissolve in water to yield ions are called electrolytes. Nonpolar molecules such as those. Its ionic nature allows it to dissolve easily in water. Which substances are most likely to undergo dissolution in water? Study with quizlet. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From www.bbc.co.uk
Which materials dissolve in water? BBC Bitesize Which Substances Dissolve In Water Check All Of The Boxes That Apply In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. Nonelectrolytes are substances that do not produce ions when. Nonpolar molecules such as those.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.
From sciencenotes.org
Is Dissolving Salt in Water a Chemical Change or a Physical Change? Which Substances Dissolve In Water Check All Of The Boxes That Apply Nonpolar molecules such as those. Study with quizlet and memorize flashcards containing terms like which of these statements would be true if the water molecule was linear. In order for a nonpolar molecule to dissolve in water, it would need to break up some of the hydrogen bonds between adjacent water. Nonelectrolytes are substances that do not produce ions when.. Which Substances Dissolve In Water Check All Of The Boxes That Apply.