Titration For Acetic Acid In Vinegar Hol Lab . the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): Assess the random error in the volume. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. demonstrate proper techniques for using `pipette, burette and volumetric flask.
from www.studocu.com
the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. Assess the random error in the volume. demonstrate proper techniques for using `pipette, burette and volumetric flask. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar.
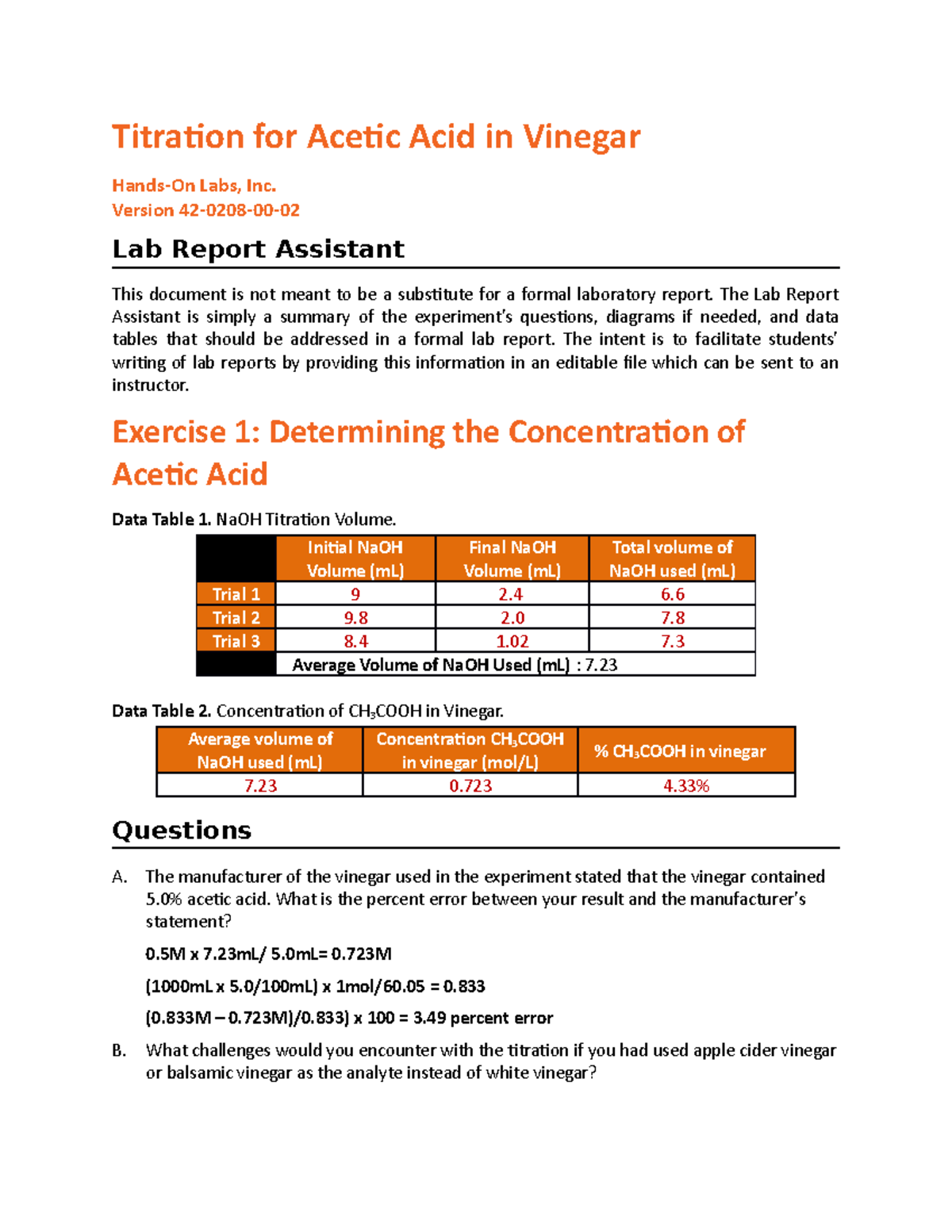
Titration for Acetic Acid in Vinegar Titration for Acetic Acid in
Titration For Acetic Acid In Vinegar Hol Lab the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. demonstrate proper techniques for using `pipette, burette and volumetric flask. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method.
From www.numerade.com
SOLVED Titlewithtopic Titration Experiment for Acetic Acid in Vinegar Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used. Titration For Acetic Acid In Vinegar Hol Lab.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Titration For Acetic Acid In Vinegar Hol Lab the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): the overall. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
Experiment_on_Determination_of_Amount_of_Acetic_Acid_in_Vinegar_by Titration For Acetic Acid In Vinegar Hol Lab the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. demonstrate proper techniques for using `pipette,. Titration For Acetic Acid In Vinegar Hol Lab.
From chem4three.blogspot.com
CHEMISTRY 11 Titration of Vinegar LAB Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using. Titration For Acetic Acid In Vinegar Hol Lab.
From chem.libretexts.org
11 Titration of Vinegar (Experiment) Chemistry LibreTexts Titration For Acetic Acid In Vinegar Hol Lab the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random. Titration For Acetic Acid In Vinegar Hol Lab.
From www.studypool.com
SOLUTION C Lab 6 Titration Of Acetic Acid In Vinegar Studypool Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): the overall purpose of this experiment was to determine the amount of acetic. Titration For Acetic Acid In Vinegar Hol Lab.
From exotlklbh.blob.core.windows.net
Determination Of Acetic Acid In Vinegar By Titration Lab Report at Titration For Acetic Acid In Vinegar Hol Lab the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. demonstrate proper techniques for using `pipette, burette and volumetric flask. in this experiment, a technique known as a. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
THE TITRATION OF ACETIC ACID IN VINEGAR.docx Titration Analytical Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. Assess the random. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. demonstrate proper techniques for using `pipette, burette and volumetric flask. Assess the random error in the volume. in. Titration For Acetic Acid In Vinegar Hol Lab.
From studylib.net
The Titration of Acetic Acid in Vinegar Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will be used to determine the concentration of acetic. Titration For Acetic Acid In Vinegar Hol Lab.
From studylib.net
Titration Lab Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in VinegarLab Report Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. the titer of sodium hydroxide is determined using. Titration For Acetic Acid In Vinegar Hol Lab.
From annie-chemistry.blogspot.com
PreAP Chemistry Blog Acetic Acid in Vinegar Titration lab Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using. Titration For Acetic Acid In Vinegar Hol Lab.
From exotlklbh.blob.core.windows.net
Determination Of Acetic Acid In Vinegar By Titration Lab Report at Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
Lab Titration of Vinegar Acid Titration Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Determination of Acetic Acid Concentration in Vinegar Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
Measuring The Amount of Acetic Acid in Vinegar by Titration With An Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. Assess the random error in the volume. the titer of sodium hydroxide is. Titration For Acetic Acid In Vinegar Hol Lab.
From www.youtube.com
Lab Determination of Acetic Acid Concentration in Vinegar using Titration For Acetic Acid In Vinegar Hol Lab the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will. Titration For Acetic Acid In Vinegar Hol Lab.
From www.numerade.com
SOLVED Titration for Acetic Acid in Vinegar Lab Report Exercise 1 Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): Assess the random error in the volume. demonstrate proper techniques for using `pipette, burette and volumetric flask. the overall. Titration For Acetic Acid In Vinegar Hol Lab.
From www.studypool.com
SOLUTION CHEM151 Phoenix Titration for Acetic Acid in Vinegar Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the overall purpose of this experiment was to determine the amount of acetic. Titration For Acetic Acid In Vinegar Hol Lab.
From www.numerade.com
SOLVED REPORT SHEET AcidBase Titration LAB 12 Concentration of Acetic Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. the titer. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. demonstrate proper techniques for using `pipette,. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Results Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will. Titration For Acetic Acid In Vinegar Hol Lab.
From www.studypool.com
SOLUTION Titration For Acetic Acid In Vinegar Studypool Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. the goal of this experiment is. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Solved Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
The Titration of Acetic Acid in Vinegar CHEM 122L General Chemistry Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. demonstrate proper techniques for using `pipette, burette and volumetric flask. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in. Titration For Acetic Acid In Vinegar Hol Lab.
From www.scribd.com
determination of acetic acid in vinegar by titration lab PDF Titration For Acetic Acid In Vinegar Hol Lab the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. Assess the random error in the volume. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. demonstrate proper techniques for using `pipette,. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. the goal of this experiment is to determine accurately the concentration of acetic acid in vinegar via. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the titer of sodium hydroxide is determined using. Titration For Acetic Acid In Vinegar Hol Lab.
From www.numerade.com
SOLVED How do I find the Concentration of Vinegar? 5mL of vinegar was Titration For Acetic Acid In Vinegar Hol Lab Assess the random error in the volume. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the goal of this experiment is. Titration For Acetic Acid In Vinegar Hol Lab.
From www.chegg.com
Titration for Acetic Acid in Vinegar Lab Report Titration For Acetic Acid In Vinegar Hol Lab the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. Assess the random error in the volume. in this experiment, a technique known as a titration will be. Titration For Acetic Acid In Vinegar Hol Lab.
From www.studocu.com
Titration for Acetic Acid in Vinegar Titration for Acetic Acid in Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. the goal. Titration For Acetic Acid In Vinegar Hol Lab.
From dokumen.tips
(PDF) Titration of Acetic Acid in Vinegar Lab Experiment DOKUMEN.TIPS Titration For Acetic Acid In Vinegar Hol Lab demonstrate proper techniques for using `pipette, burette and volumetric flask. the titer of sodium hydroxide is determined using potassium hydrogen phthalate as a primary standard (khp): in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. in this experiment, a technique known as a titration. Titration For Acetic Acid In Vinegar Hol Lab.
From www.studocu.com
Vinegar Titration LAB Francis TITRATION OF ACETIC ACID IN VINEGAR Titration For Acetic Acid In Vinegar Hol Lab in this experiment, a technique known as a titration will be used to determine the concentration of acetic acid in vinegar. Assess the random error in the volume. the overall purpose of this experiment was to determine the amount of acetic acid, which is what was done using the titration method. in this experiment, a technique known. Titration For Acetic Acid In Vinegar Hol Lab.