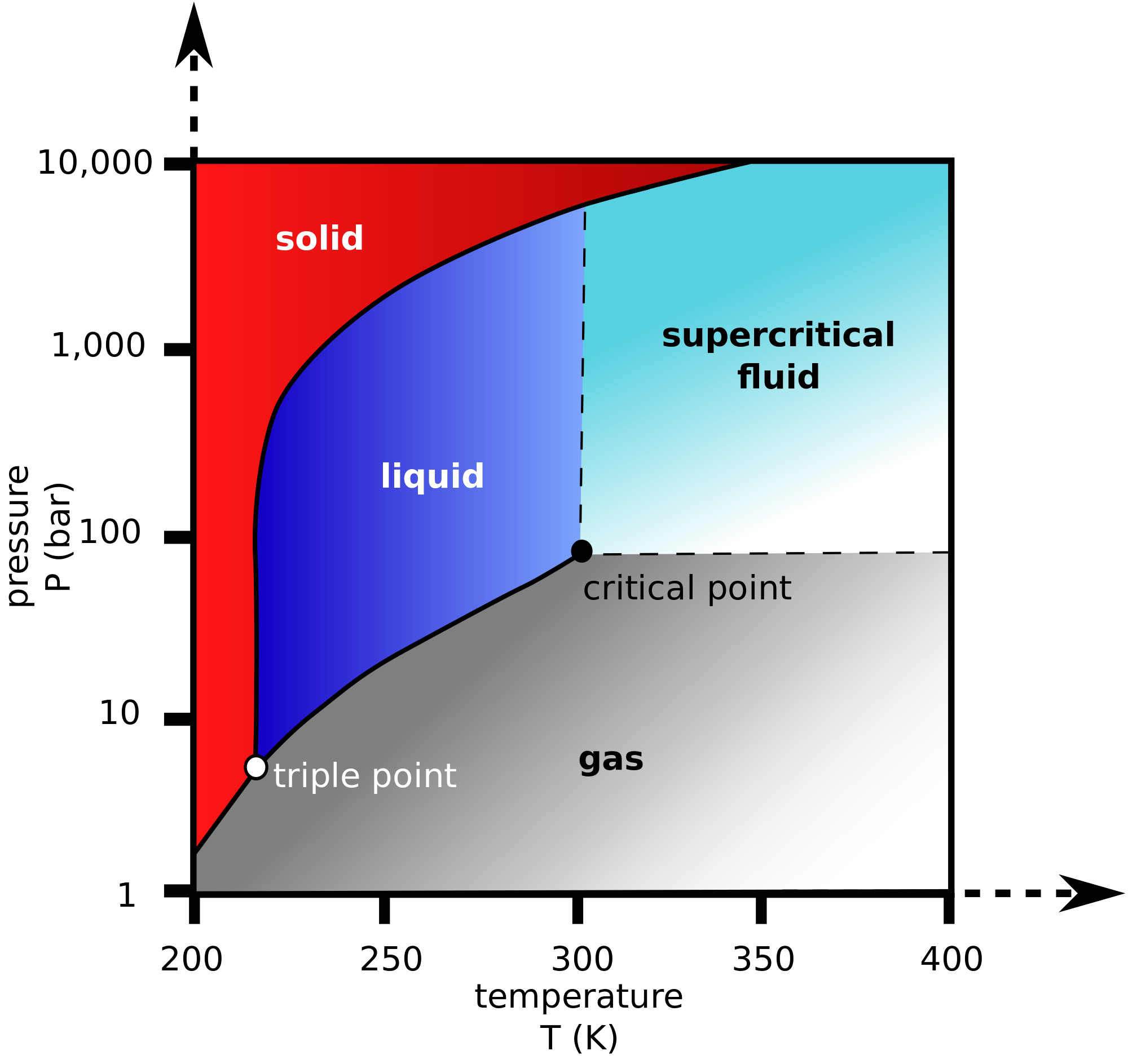
Vapor Pressure Definition Science . vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature, how it. Learn how vapor pressure depends on temperature,. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. vapor pressure is the partial pressure of a gas above a liquid in equilibrium.
from socratic.org
vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. Learn how vapor pressure depends on temperature,. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature, how it. vapor pressure is the partial pressure of a gas above a liquid in equilibrium.
What is the relation between critical temperature and boiling point or
Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature, how it. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. Learn how vapor pressure depends on temperature,.
From study.com
Vapor Pressure Definition, Formula & Examples Lesson Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature,. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases,. Vapor Pressure Definition Science.
From www.pipingengineer.org
Centrifugal Pump Terminology The Piping Engineering World Vapor Pressure Definition Science Learn how vapor pressure depends on temperature,. Learn how vapor pressure depends on temperature, how it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is. Vapor Pressure Definition Science.
From www.youtube.com
Evaporation, Vapor Pressure and Boiling YouTube Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapor pressure depends on temperature, how it. vapor pressure is the pressure exerted by the gas above a liquid or. Vapor Pressure Definition Science.
From www.omnicalculator.com
Vapor Pressure of Water Calculator Definition Formulas Vapor Pressure Definition Science learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour with its. Vapor Pressure Definition Science.
From flatworldknowledge.lardbucket.org
Vapor Pressure Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. . Vapor Pressure Definition Science.
From socratic.org
What is the relation between critical temperature and boiling point or Vapor Pressure Definition Science Learn how vapor pressure depends on temperature,. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour with its condensed phases in a. Vapor Pressure Definition Science.
From courses.lumenlearning.com
Vapor Pressure of Electrolyte Solutions Introduction to Chemistry Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. Learn how vapour pressure depends. Vapor Pressure Definition Science.
From www.youtube.com
vapour pressure,definition,unit, factors YouTube Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Solution properties PowerPoint Presentation, free download ID Vapor Pressure Definition Science vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature,. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases,. Vapor Pressure Definition Science.
From www.pinterest.ph
Vapor Pressure Easy Science Física Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Pressure Definition Science vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature, how it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapour pressure is the pressure exerted by a. Vapor Pressure Definition Science.
From chem.libretexts.org
13.6 Vapor Pressures of Solutions Chemistry LibreTexts Vapor Pressure Definition Science vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. vapor pressure is the partial pressure of a gas above. Vapor Pressure Definition Science.
From www.slideshare.net
Vapor pressure and boiling Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which.. Vapor Pressure Definition Science.
From www.slideserve.com
PPT VAPOR PRESSURE OF WATER PowerPoint Presentation, free download Vapor Pressure Definition Science Learn how vapor pressure depends on temperature, how it. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapor pressure depends on temperature,. Learn how vapour pressure. Vapor Pressure Definition Science.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. . Vapor Pressure Definition Science.
From www.youtube.com
Equilibrium vapor pressure Meaning YouTube Vapor Pressure Definition Science Learn how vapor pressure depends on temperature,. Learn how vapor pressure depends on temperature, how it. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapour pressure is the pressure. Vapor Pressure Definition Science.
From inspiritvr.com
Vapor Pressure Lowering Study Guide Inspirit Learning Inc Vapor Pressure Definition Science Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Liquids, Solids, and Intermolecular Forces PowerPoint Vapor Pressure Definition Science Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor. Vapor Pressure Definition Science.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapour pressure is. Vapor Pressure Definition Science.
From www.thoughtco.com
Vapor Definition Chemistry Glossary Vapor Pressure Definition Science learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour with its. Vapor Pressure Definition Science.
From chem.libretexts.org
Chapter 11.4 Vapor Pressure Chemistry LibreTexts Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. Learn how vapor pressure depends on temperature,. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapor pressure is the pressure exerted by the gas above a liquid or a solid. Vapor Pressure Definition Science.
From www.youtube.com
Vapor pressure versus temperature relations of common elements YouTube Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature, how it. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure. Vapor Pressure Definition Science.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. Learn how vapor pressure depends on temperature, how it. Learn how vapor pressure depends on temperature,. learn about vapor pressure, the partial. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which.. Vapor Pressure Definition Science.
From www.britannica.com
Pressure Definition, Measurement, & Types Britannica Vapor Pressure Definition Science learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature,. Learn how vapor pressure depends on temperature, how it. vapour pressure is. Vapor Pressure Definition Science.
From www.youtube.com
Vapor pressure example Chemistry Khan Academy YouTube Vapor Pressure Definition Science Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. learn about vapor pressure, the partial pressure of a vapor. Vapor Pressure Definition Science.
From saylordotorg.github.io
Vapor Pressure Vapor Pressure Definition Science learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at. Vapor Pressure Definition Science.
From www.geeksforgeeks.org
What is Vaporization? Vapor Pressure Definition Science Learn how vapor pressure depends on temperature,. Learn how vapor pressure depends on temperature, how it. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by the gas above. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Why . . . PowerPoint Presentation, free download ID2250075 Vapor Pressure Definition Science vapor pressure is the partial pressure of a gas above a liquid in equilibrium. Learn how vapor pressure depends on temperature, how it. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Vapor Pressure PowerPoint Presentation, free download ID5080574 Vapor Pressure Definition Science Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and videos. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapor pressure is the pressure exerted by. Vapor Pressure Definition Science.
From thechemistrynotes.com
Vapor Pressure of LiquidLiquid Solution Vapor Pressure Definition Science learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapor pressure depends on temperature,. vapor pressure is the partial pressure of a gas above a liquid in equilibrium. vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or. Vapor Pressure Definition Science.
From www.tec-science.com
Specific latent heat of condensation tecscience Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. Learn how vapor pressure depends on temperature, how it. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor. Vapor Pressure Definition Science.
From www.slideserve.com
PPT Evaluation of VOC Definition Based on Vapor Pressure PowerPoint Vapor Pressure Definition Science vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapor pressure depends on temperature,. vapour pressure is the pressure exerted by a vapour in. Vapor Pressure Definition Science.
From www.ck12.org
VaporPressure Lowering (Raoult's Law) ΔP = X[B]P°[A] Example 1 Vapor Pressure Definition Science vapor pressure is the pressure exerted by the gas above a liquid or a solid in equilibrium with it. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's law, which. Learn how vapour pressure depends on temperature, nature of liquid, raoult's law and boiling point with examples and. Vapor Pressure Definition Science.
From www.youtube.com
Vaporization and Vapor Pressure YouTube Vapor Pressure Definition Science vapour pressure is the pressure exerted by a vapour in equilibrium with its liquid or solid form. vapour pressure is the pressure exerted by a vapour with its condensed phases in a closed system at a given temperature. learn about vapor pressure, the partial pressure of a vapor in dynamic equilibrium with its condensed phases, and raoult's. Vapor Pressure Definition Science.