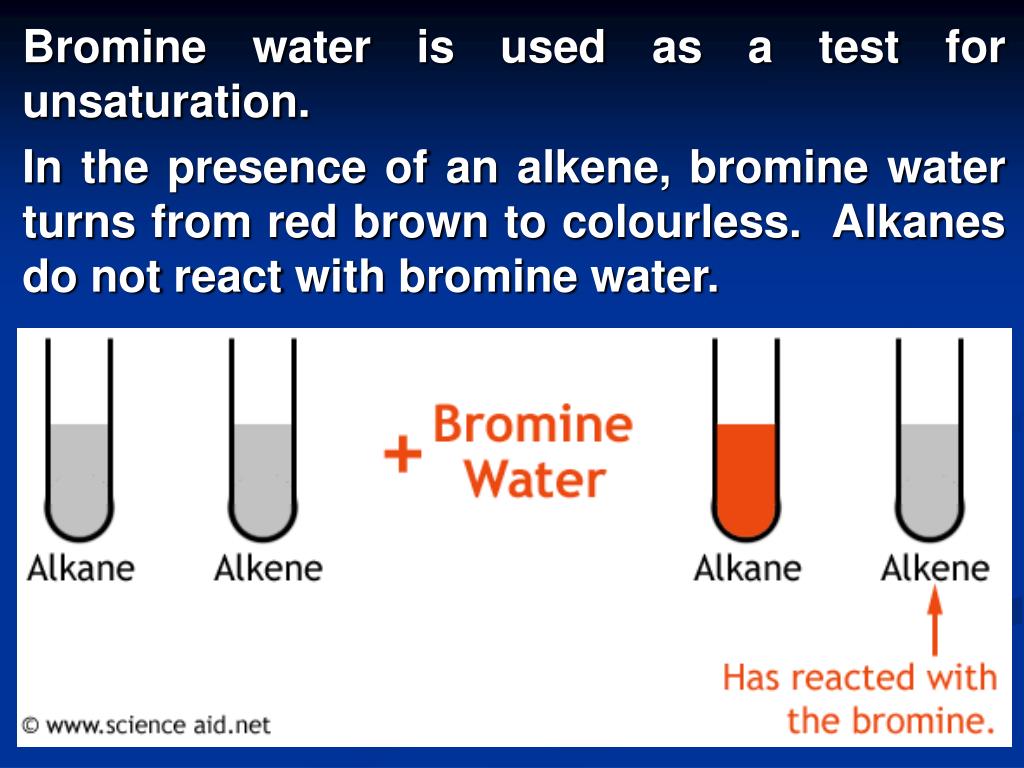
Bromine Water Go Colourless . Why does bromine become colourless in the alkene (but not the alkane)? When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. Bromine water is an orange coloured solution. To do this, bromine water is used. In halogenation (a type of reaction) of alkenes,. The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken. If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. To know how this works, we need to look at the equations. There is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken with an alkane the solution remains orange. Bromine water is an orange coloured solution. There is no change when bromine water is mixed with an alkane;
from www.slideserve.com
Bromine water is an orange coloured solution. When bromine water is shaken with an alkane the solution remains orange. The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken. Bromine water is an orange coloured solution. In halogenation (a type of reaction) of alkenes,. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. There is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane.
PPT Organic Chemistry PowerPoint Presentation, free download ID3294220
Bromine Water Go Colourless Why does bromine become colourless in the alkene (but not the alkane)? The bromine water becomes colourless when it is mixed with an alkene When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. To do this, bromine water is used. Bromine water is an orange coloured solution. The bromine water becomes colourless when it is mixed with an alkene In halogenation (a type of reaction) of alkenes,. To know how this works, we need to look at the equations. Why does bromine become colourless in the alkene (but not the alkane)? Bromine water is an orange coloured solution. When bromine water is shaken. If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. There is no change when bromine water is mixed with an alkane; When bromine water is shaken with an alkane the solution remains orange. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. There is no change when bromine water is mixed with an alkane;
From slideplayer.com
Just remember…. CHEMISTRY IS GREAT!!! ppt download Bromine Water Go Colourless When bromine water is shaken with an alkane the solution remains orange. To know how this works, we need to look at the equations. When bromine water is shaken. The bromine water becomes colourless when it is mixed with an alkene The bromine water becomes colourless when it is mixed with an alkene Bromine water is an orange coloured solution.. Bromine Water Go Colourless.
From present5.com
19 03 2018 Chemistry Unit C 1 Chemistry in Bromine Water Go Colourless If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. Bromine water is an orange coloured solution. When bromine water is shaken. To do this, bromine water is used. Bromine water is an orange coloured solution. To know how this works, we need to look at the equations. When bromine. Bromine Water Go Colourless.
From www.biophlox.com
Buy Bromine Water get price for lab equipment Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene Bromine water is an orange coloured solution. The bromine water becomes colourless when it is mixed with an alkene If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. There is no change when bromine water is mixed. Bromine Water Go Colourless.
From socratic.org
Question 57f2d Socratic Bromine Water Go Colourless Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine water becomes colourless when it is mixed with an alkene The bromine water becomes colourless when it is mixed with an alkene In halogenation (a type of reaction) of alkenes,. To do this, bromine water is used.. Bromine Water Go Colourless.
From exoiawwlq.blob.core.windows.net
Use Bromine Water Test at Bryan Green blog Bromine Water Go Colourless When bromine water is shaken. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. There is no change when bromine water is mixed with an alkane; To do this, bromine water is used. Bromine water is an orange coloured solution. Bromine water is. Bromine Water Go Colourless.
From slideplayer.com
Revision Notes C1. ppt download Bromine Water Go Colourless In halogenation (a type of reaction) of alkenes,. The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken. When bromine water is shaken with an alkane the solution remains orange. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you. Bromine Water Go Colourless.
From alchetron.com
Bromine water Alchetron, The Free Social Encyclopedia Bromine Water Go Colourless When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. Bromine water is an orange coloured solution. Why does bromine become colourless in the alkene (but not the alkane)? Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Water Go Colourless.
From www.shutterstock.com
Lo mejor en la categoría «Ethyl glycol test» de imágenes, fotos de Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene There is no change when bromine water is mixed with an alkane; When bromine water is shaken. To do this, bromine water is used. The bromine water becomes colourless when it is mixed with an alkene If you shake an alkene with bromine water (or bubble a gaseous. Bromine Water Go Colourless.
From askfilo.com
Bromine water reacts with aniline to give Filo Bromine Water Go Colourless To do this, bromine water is used. To know how this works, we need to look at the equations. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. There is no change when bromine water is mixed with an alkane; Bromine water is. Bromine Water Go Colourless.
From alchetron.com
Bromine water Alchetron, The Free Social Encyclopedia Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene Bromine water is an orange coloured solution. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. Bromine water is an orange coloured solution. To know how this works, we need to. Bromine Water Go Colourless.
From www.sciencephoto.com
Decolourised bromine water Stock Image C029/0948 Science Photo Bromine Water Go Colourless There is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Bromine water is an orange coloured solution. In halogenation (a type of reaction) of alkenes,.. Bromine Water Go Colourless.
From www.flickriver.com
Bromine water a photo on Flickriver Bromine Water Go Colourless To do this, bromine water is used. There is no change when bromine water is mixed with an alkane; If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. When bromine water is shaken. When bromine water is added to an alkane, it will remain as an orange solution as. Bromine Water Go Colourless.
From www.youtube.com
Bromine Water Test For Unsaturation Red Brown Solution Practical Bromine Water Go Colourless Why does bromine become colourless in the alkene (but not the alkane)? There is no change when bromine water is mixed with an alkane; When bromine water is shaken. The bromine water becomes colourless when it is mixed with an alkene There is no change when bromine water is mixed with an alkane; In halogenation (a type of reaction) of. Bromine Water Go Colourless.
From www.youtube.com
Test for Unsaturation Bromine Test YouTube Bromine Water Go Colourless To know how this works, we need to look at the equations. There is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. When bromine water. Bromine Water Go Colourless.
From www.youtube.com
Why Is Bromine Not Used In Pools? YouTube Bromine Water Go Colourless In halogenation (a type of reaction) of alkenes,. Bromine water is an orange coloured solution. There is no change when bromine water is mixed with an alkane; When bromine water is shaken with an alkane the solution remains orange. To know how this works, we need to look at the equations. When bromine water is shaken. When bromine water is. Bromine Water Go Colourless.
From www.numerade.com
QUESTION 4 Two test tubes A and B contain hex1ene and hexane Bromine Water Go Colourless Bromine water is an orange coloured solution. The bromine water becomes colourless when it is mixed with an alkene The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken. Why does bromine become colourless in the alkene (but not the alkane)? To know how this works, we need to look at the equations.. Bromine Water Go Colourless.
From ar.inspiredpencil.com
Bromine Water Bromine Water Go Colourless When bromine water is shaken. Why does bromine become colourless in the alkene (but not the alkane)? There is no change when bromine water is mixed with an alkane; When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. To do this, bromine water. Bromine Water Go Colourless.
From www.sciencephoto.com
Bromine test for alkene Stock Image A500/0454 Science Photo Library Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene There is no change when bromine water is mixed with an alkane; When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. If you shake an alkene with bromine water (or bubble. Bromine Water Go Colourless.
From www.edenhottubs.co.uk
Bromine Chemical Pack Eden Hot Tubs Hot Tubs Cornwall Bromine Water Go Colourless In halogenation (a type of reaction) of alkenes,. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The bromine water becomes colourless when it is mixed with an alkene When bromine water is shaken with an alkane the solution remains orange. When bromine water is shaken. There is. Bromine Water Go Colourless.
From www.slideserve.com
PPT Organic Chemistry PowerPoint Presentation, free download ID3294220 Bromine Water Go Colourless Why does bromine become colourless in the alkene (but not the alkane)? There is no change when bromine water is mixed with an alkane; The bromine water becomes colourless when it is mixed with an alkene In halogenation (a type of reaction) of alkenes,. To do this, bromine water is used. To know how this works, we need to look. Bromine Water Go Colourless.
From ar.inspiredpencil.com
Uses Of Bromine Bromine Water Go Colourless When bromine water is shaken. There is no change when bromine water is mixed with an alkane; Bromine water is an orange coloured solution. If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. Bromine water is an orange coloured solution. Why does bromine become colourless in the alkene (but. Bromine Water Go Colourless.
From www.slideserve.com
PPT 2.7 Physical Properties of Alkenes PowerPoint Presentation, free Bromine Water Go Colourless There is no change when bromine water is mixed with an alkane; If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. When bromine water is shaken. Why does bromine become colourless in the alkene (but not the alkane)? Bromine water is an orange coloured solution. To know how this. Bromine Water Go Colourless.
From diyquickly.com
How to Lower Bromine in Hot Tub Described in 10 Steps (2024) Bromine Water Go Colourless There is no change when bromine water is mixed with an alkane; Bromine water is an orange coloured solution. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. In halogenation (a type of reaction) of alkenes,. Why does bromine become colourless in the alkene (but not the alkane)?. Bromine Water Go Colourless.
From www.youtube.com
Experiment of Bromine Water Test🔥 Organic Chemistry Imran Sir Bromine Water Go Colourless If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. To know how this works, we need to look at the equations. In halogenation (a type of reaction) of alkenes,. There is no change when bromine water is mixed with an alkane; Alkenes react in the cold with pure liquid. Bromine Water Go Colourless.
From slideplayer.com
1.5 b Learning apply knowledge of oxidation and reduction to Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene There is no change when bromine water is mixed with an alkane; To do this, bromine water is used. Bromine water is an orange coloured solution. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon. Bromine Water Go Colourless.
From alannameowarias.blogspot.com
Colour of Bromine Water Bromine Water Go Colourless Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. When bromine water is shaken. Bromine water is an orange coloured solution. The bromine water becomes colourless when it. Bromine Water Go Colourless.
From www.chemistore.co.za
Bromine water, 500ml Bromine Water Go Colourless There is no change when bromine water is mixed with an alkane; In halogenation (a type of reaction) of alkenes,. To know how this works, we need to look at the equations. Why does bromine become colourless in the alkene (but not the alkane)? If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water),. Bromine Water Go Colourless.
From quintensrtate.blogspot.com
Colour of Bromine Water QuintensrTate Bromine Water Go Colourless Bromine water is an orange coloured solution. When bromine water is shaken. To know how this works, we need to look at the equations. There is no change when bromine water is mixed with an alkane; If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. When bromine water is. Bromine Water Go Colourless.
From www.linstitute.net
EDEXCEL IGCSE CHEMISTRY DOUBLE SCIENCE 复习笔记:4.4.2 Bromine & Alkenes Bromine Water Go Colourless To do this, bromine water is used. The bromine water becomes colourless when it is mixed with an alkene There is no change when bromine water is mixed with an alkane; Bromine water is an orange coloured solution. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon. Bromine Water Go Colourless.
From dir.indiamart.com
Bromine Water Bromide Bromate Solution Latest Price, Manufacturers Bromine Water Go Colourless If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. In halogenation (a type of reaction) of alkenes,. When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. To know how this works, we. Bromine Water Go Colourless.
From slideplayer.com
Revision Notes C1. ppt download Bromine Water Go Colourless When bromine water is shaken with an alkane the solution remains orange. Why does bromine become colourless in the alkene (but not the alkane)? To know how this works, we need to look at the equations. Bromine water is an orange coloured solution. In halogenation (a type of reaction) of alkenes,. If you shake an alkene with bromine water (or. Bromine Water Go Colourless.
From www.doubtnut.com
[Odia] Phenol treated with excess of bromine water gives Bromine Water Go Colourless There is no change when bromine water is mixed with an alkane; To know how this works, we need to look at the equations. The bromine water becomes colourless when it is mixed with an alkene Bromine water is an orange coloured solution. In halogenation (a type of reaction) of alkenes,. Bromine water is an orange coloured solution. When bromine. Bromine Water Go Colourless.
From byjus.com
How do you test for an alkene? Bromine Water Go Colourless The bromine water becomes colourless when it is mixed with an alkene If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. When bromine water is shaken. There is no change when bromine water is mixed with an alkane; Bromine water is an orange coloured solution. When bromine water is. Bromine Water Go Colourless.
From slidetodoc.com
AS Chemistry Alkenes Alkenes Lesson objective To know Bromine Water Go Colourless If you shake an alkene with bromine water (or bubble a gaseous alkene through bromine water), the solution becomes colourless. There is no change when bromine water is mixed with an alkane; Bromine water is an orange coloured solution. To know how this works, we need to look at the equations. The bromine water becomes colourless when it is mixed. Bromine Water Go Colourless.
From www.indiamart.com
500ml Indochem Bromine Water at Rs 126 Liquid Chemical in Hapur ID Bromine Water Go Colourless When bromine water is added to an alkane, it will remain as an orange solution as alkanes do not have double carbon bonds (c=c) so the. Why does bromine become colourless in the alkene (but not the alkane)? The bromine water becomes colourless when it is mixed with an alkene Bromine water is an orange coloured solution. In halogenation (a. Bromine Water Go Colourless.