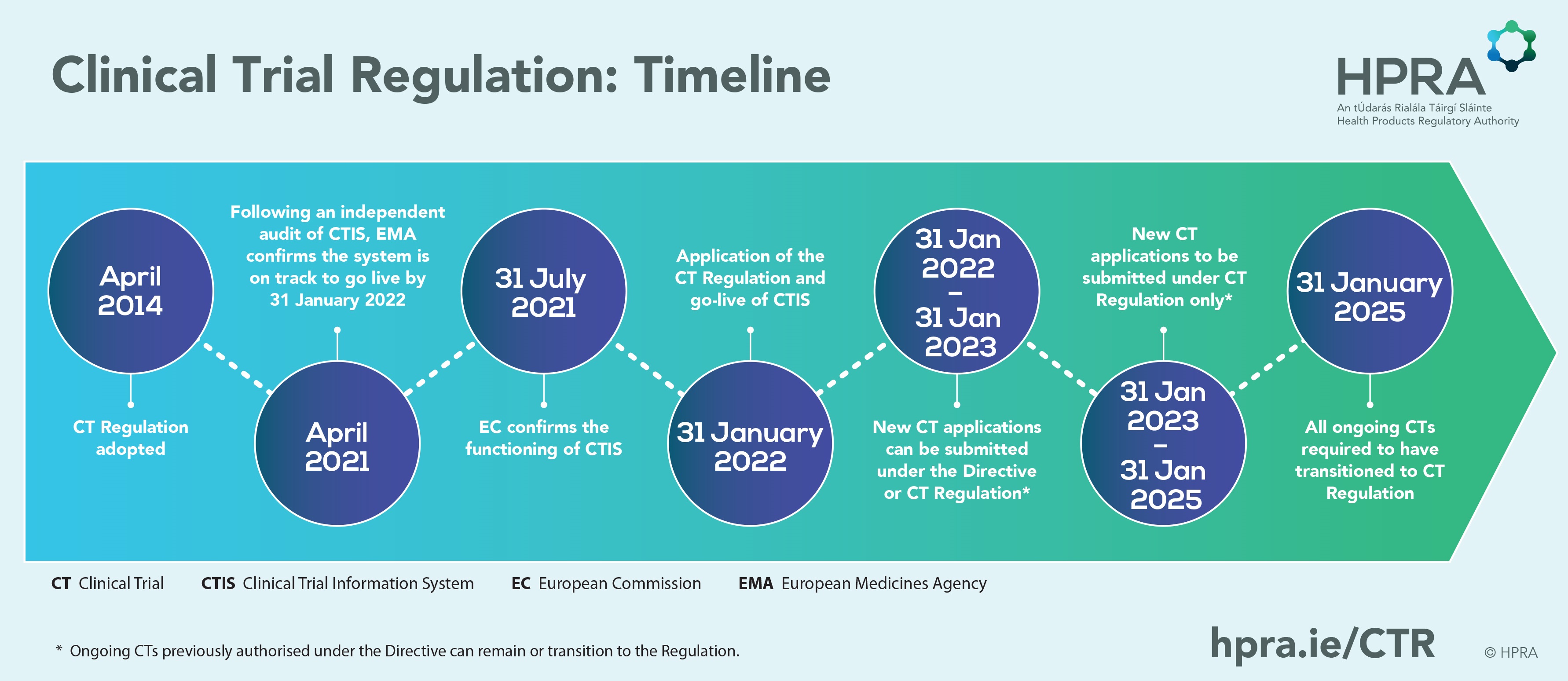
Ctr Regulation . Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Find out how to use the clinical trials information system (ctis) to. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Transition period from directive 2001/20/ec to regulation (eu) no. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. What is the aim of the regulation? Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022.
from www.hpra.ie
The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Transition period from directive 2001/20/ec to regulation (eu) no. Find out how to use the clinical trials information system (ctis) to. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. What is the aim of the regulation? Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022.
Clinical Trials Regulation
Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Transition period from directive 2001/20/ec to regulation (eu) no. Find out how to use the clinical trials information system (ctis) to. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. What is the aim of the regulation?
From pharmexcel-cro.com
New EU Clinical Trial Regulation Clinical Trial Services, UK PHARMExcel Ctr Regulation Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. What is the aim of the regulation? Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. The european union clinical trials regulation (regulation (eu) no 536/2014). Ctr Regulation.
From www.cell.com
The role of ethylene in plant temperature stress response Trends in Ctr Regulation Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Regulation (eu) no 536/2014 of the european parliament and of the council of. Ctr Regulation.
From www.precisionformedicine.com
Implications of EUCTR Regulation 536/2014 Precision For Medicine Ctr Regulation The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022.. Ctr Regulation.
From www.datenschutz-notizen.de
Processing Personal Data in the Context of the Clinical Trial Ctr Regulation Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. What is the aim of the regulation? Transition period from directive 2001/20/ec to regulation (eu) no. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Regulation (eu) no 536/2014 on clinical. Ctr Regulation.
From fusion-pharma-limited.com
Clinical Trial Regulation (CTR) A Closer Look Fusion Pharma Ctr Regulation Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Transition period from directive 2001/20/ec to regulation (eu) no. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu) no 536/2014 of the european parliament and of the council of 16. Ctr Regulation.
From lumisinternational.com
Transitioning to the Clinical Trials Regulation 536/2014 (CTR) Key Ctr Regulation Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Find out how to use the clinical trials information system (ctis) to. Clinical trials in the eu are governed by the clinical trials. Ctr Regulation.
From gcpcentral.com
Implementing the EU CTR Publishing Clinical Trial Reports and Ctr Regulation Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal. Ctr Regulation.
From www.researchgate.net
Study design and methodology. CT clinical trial; PCP primary care Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. What is the aim of the regulation? The. Ctr Regulation.
From pci.com
EU Clinical Trials Regulation (CTR) No 536/2014 Are You Prepared Ctr Regulation Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Find out how to use the clinical trials information system (ctis) to. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the. Ctr Regulation.
From www.milestoneloc.com
ICF Translation Importance, Requirements & Best Practices Ctr Regulation Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. What is the aim of. Ctr Regulation.
From lifesciences.transperfect.com
Assessing Readiness for the EU Clinical Trials Regulation (CTR) Five Ctr Regulation What is the aim of the regulation? Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Find out how to use the clinical trials information system (ctis) to. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu) no 536/2014. Ctr Regulation.
From lsacademy.com
Training on Safety in Clinical Trial Regulation (CTR) No 536/2014 LS Ctr Regulation Find out how to use the clinical trials information system (ctis) to. Transition period from directive 2001/20/ec to regulation (eu) no. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu). Ctr Regulation.
From www.veeva.com
Assessing the Organizational Impact of EMA's Clinical Trial Regulation Ctr Regulation What is the aim of the regulation? The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Learn. Ctr Regulation.
From vdocuments.mx
Regulation of CTR2 mRNA by the nonsensemediated mRNA decay pathway Ctr Regulation The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Find out how to use the clinical trials information system (ctis) to. Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 of the european parliament and of the. Ctr Regulation.
From rednucleus.com
Enabling EU CTR Compliance Readiness Red Nucleus Ctr Regulation Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline. Ctr Regulation.
From www.nrecoffice.ie
CTR National Requirements Part II NREC Ctr Regulation Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Find out how to use the clinical trials information system (ctis) to. The european union clinical trials regulation (regulation (eu) no 536/2014) is. Ctr Regulation.
From boydconsultants.com
Preparing for the Clinical Trials Regulation (CTR) Boyds Ctr Regulation Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. What is the aim of the regulation? Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Find out how to use the clinical trials information system (ctis) to. Learn about the eu legislation that harmonises and simplifies. Ctr Regulation.
From vietstar-research.com
Clinical trial regulation in EU Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. What is the aim of the regulation? Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn how the clinical trials regulation (ctr). Ctr Regulation.
From www.researchgate.net
CTR9 regulated genes are subjected to regulation by PRC2 Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for. Ctr Regulation.
From tlfc.com.au
CTRS Regulations 2022 B&W Tisher Liner FC Law Ctr Regulation Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. What is the aim of the regulation? Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products. Ctr Regulation.
From www.pharmaceutical-technology.com
New EUCTR and what it means for Part I clinical trial documentation Ctr Regulation What is the aim of the regulation? Transition period from directive 2001/20/ec to regulation (eu) no. Find out how to use the clinical trials information system (ctis) to. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of. Ctr Regulation.
From www.researchgate.net
The Ctr9mediated regulation of DAT does not require the SH2 domain of Ctr Regulation Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. The. Ctr Regulation.
From www.tracercro.com
Regulation EU No 536 2014 Imaging CRO TRACER Ctr Regulation Find out how to use the clinical trials information system (ctis) to. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn how the clinical trials regulation (ctr) and the clinical trials. Ctr Regulation.
From www.slideserve.com
PPT Regulation of Gene Expression by Eukaryotes PowerPoint Ctr Regulation Find out how to use the clinical trials information system (ctis) to. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on. Ctr Regulation.
From allevents.in
EUCTR New regulations are here Are you ready for the changes Ctr Regulation The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Regulation (eu) no 536/2014 of the european parliament and of the council of. Ctr Regulation.
From www.semanticscholar.org
Figure 2 from Calcitonin generelated peptide physiology and Ctr Regulation The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu.. Ctr Regulation.
From www.hpra.ie
Clinical Trials Regulation Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Find out how to use the clinical trials information system (ctis) to. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Regulation (eu) no 536/2014 of the european. Ctr Regulation.
From www.clinicaltrialsarena.com
EU Clinical Trial Regulation The Long Path to Implementation Ctr Regulation Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Find out how to use the clinical trials information system (ctis) to. Transition period from directive 2001/20/ec to regulation (eu) no. Learn about. Ctr Regulation.
From www.researchgate.net
The Ctr9mediated regulation of DAT does not require the SH2 domain of Ctr Regulation Find out how to use the clinical trials information system (ctis) to. Clinical trials in the eu are governed by the clinical trials regulation, which became effective on 31 january 2022. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. What is the aim of the regulation? Regulation (eu) no 536/2014 of the european parliament and. Ctr Regulation.
From globalforum.diaglobal.org
EU Clinical Trial Regulation Get Ready to Adapt! Ctr Regulation Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. What is the aim of the regulation? Learn how the clinical trials regulation (ctr) and the clinical. Ctr Regulation.
From boydconsultants.com
The Clinical Trials Regulation and the Clinical Trials Information Ctr Regulation Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. The european union clinical trials regulation (regulation (eu) no 536/2014) is the legislation relating to the conduct of clinical trials of investigational medicinal products within the european union. Learn about the eu legislation that harmonises and. Ctr Regulation.
From tlfc.com.au
Regulations CTRS Tisher Liner FC Law Ctr Regulation Find out how to use the clinical trials information system (ctis) to. What is the aim of the regulation? Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for. Ctr Regulation.
From www.actrials.com
ADVANCED CLINICAL TRIALS THE CLINICAL TRIAL PROCESS IMPENDING CHANGES Ctr Regulation Find out how to use the clinical trials information system (ctis) to. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Transition period from directive 2001/20/ec to regulation (eu) no. Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise. Ctr Regulation.
From www.nrecoffice.ie
Clinical trial applications must now be submitted through CTIS NREC Ctr Regulation Learn how the clinical trials regulation (ctr) and the clinical trials information system (ctis) harmonise and streamline the. Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 on clinical trials on medicinal products for human use. Learn about the eu legislation that harmonises and simplifies the assessment and supervision of clinical trials across the eu. Find. Ctr Regulation.
From www.researchgate.net
Effects of ctr6 overexpression on cell growth and regulation of the Ctr Regulation Transition period from directive 2001/20/ec to regulation (eu) no. Regulation (eu) no 536/2014 of the european parliament and of the council of 16 april 2014 on clinical trials on medicinal products for human. Find out how to use the clinical trials information system (ctis) to. Clinical trials in the eu are governed by the clinical trials regulation, which became effective. Ctr Regulation.