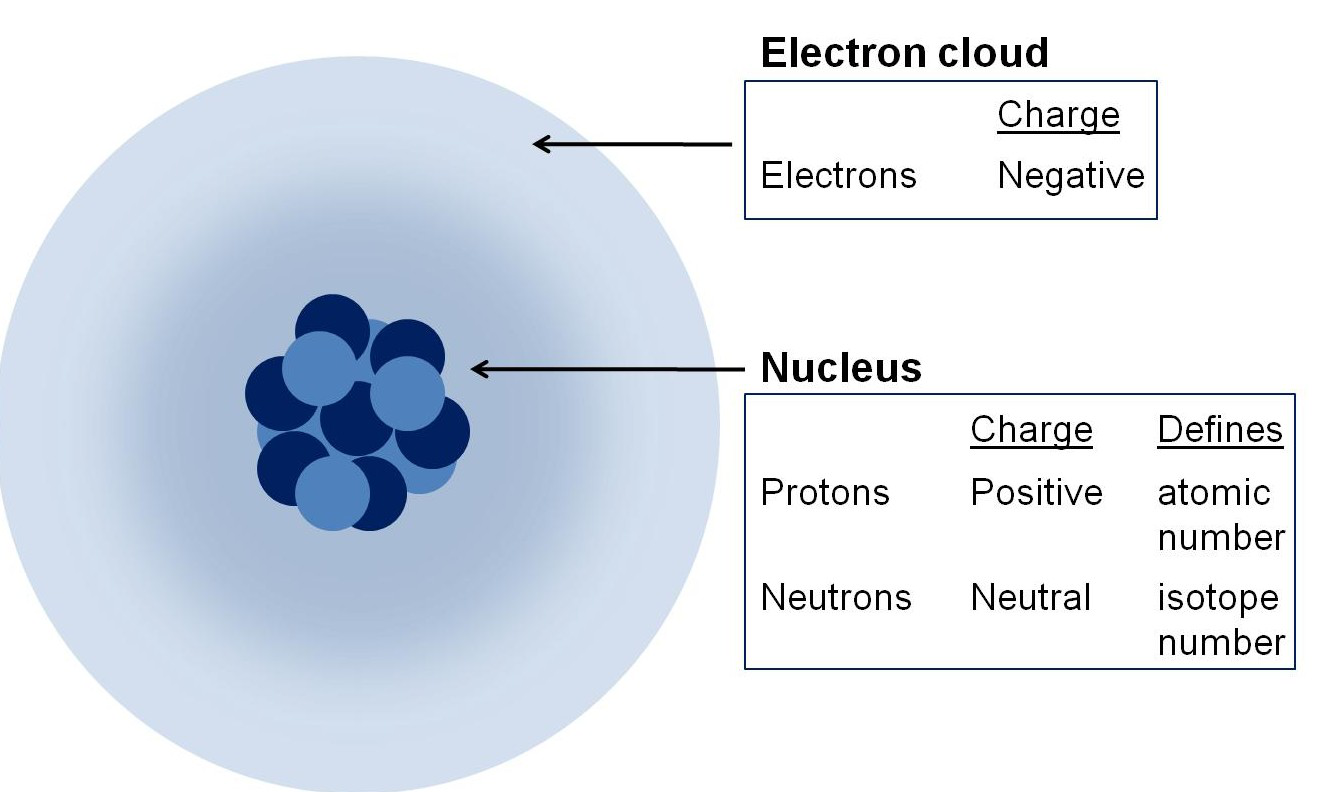
Does An Atom Nucleus Have A Positive Charge . protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. knowing that atoms are electrically neutral, j. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. Nuclear composition determines an atom’s element (number of protons) and isotope (number of. Every atom has no overall charge (neutral). the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. Thomson postulated that there must be a positive charge as well. the nucleus has a positive electrical charge. the nucleus has an overall positive charge as it contains the protons.
from data.allenai.org
the nucleus has an overall positive charge as it contains the protons. Nuclear composition determines an atom’s element (number of protons) and isotope (number of. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. Every atom has no overall charge (neutral). Thomson postulated that there must be a positive charge as well. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. the nucleus has a positive electrical charge. knowing that atoms are electrically neutral, j. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric.
inside the atom (lesson 0772) TQA explorer
Does An Atom Nucleus Have A Positive Charge protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. the nucleus has an overall positive charge as it contains the protons. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has a positive electrical charge. knowing that atoms are electrically neutral, j. Thomson postulated that there must be a positive charge as well. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. Every atom has no overall charge (neutral). Nuclear composition determines an atom’s element (number of protons) and isotope (number of. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles.
From www.sciencefacts.net
Atom Definition, Structure & Parts with Labeled Diagram Does An Atom Nucleus Have A Positive Charge an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. knowing that atoms are electrically neutral, j. the nucleus has an overall positive charge as it contains the protons. Nuclear composition determines an atom’s element (number of protons) and isotope (number of. the nucleus has a positive electrical charge. Thomson. Does An Atom Nucleus Have A Positive Charge.
From www.electronicsandyou.com
Structure of an Atom Structure & Use of Electron & Proton in Electronics Does An Atom Nucleus Have A Positive Charge Nuclear composition determines an atom’s element (number of protons) and isotope (number of. Thomson postulated that there must be a positive charge as well. knowing that atoms are electrically neutral, j. Every atom has no overall charge (neutral). the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus. Does An Atom Nucleus Have A Positive Charge.
From spmscience.blog.onlinetuition.com.my
(A) The composition of the Nucleus SPM Science Does An Atom Nucleus Have A Positive Charge Every atom has no overall charge (neutral). the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has an overall positive charge as it contains the protons. Thomson postulated that there must be a positive charge as well. the nucleus has a positive electrical charge. Nuclear composition determines. Does An Atom Nucleus Have A Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Does An Atom Nucleus Have A Positive Charge the nucleus has an overall positive charge as it contains the protons. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. Thomson postulated that there must be a positive charge as well. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. an atom. Does An Atom Nucleus Have A Positive Charge.
From www.nagwa.com
Question Video Recalling the Name of the Positively Charged Particles Does An Atom Nucleus Have A Positive Charge the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has a positive electrical charge. Thomson postulated that there must be a positive charge as well. the nucleus has an overall positive charge as it contains the protons. Nuclear composition determines an atom’s element (number of protons) and. Does An Atom Nucleus Have A Positive Charge.
From www.expii.com
Atoms — Definition & Overview Expii Does An Atom Nucleus Have A Positive Charge as their names suggest, protons have a positive electrical charge, while neutrons are electrically. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. knowing that atoms are electrically. Does An Atom Nucleus Have A Positive Charge.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID5877005 Does An Atom Nucleus Have A Positive Charge Thomson postulated that there must be a positive charge as well. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged. Does An Atom Nucleus Have A Positive Charge.
From spmphysics.onlinetuition.com.my
The Composition of the Nucleus SPM Physics Form 4/Form 5 Revision Notes Does An Atom Nucleus Have A Positive Charge Every atom has no overall charge (neutral). the nucleus has a positive electrical charge. Thomson postulated that there must be a positive charge as well. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. Nuclear composition. Does An Atom Nucleus Have A Positive Charge.
From data.allenai.org
inside the atom (lesson 0772) TQA explorer Does An Atom Nucleus Have A Positive Charge Every atom has no overall charge (neutral). the nucleus has an overall positive charge as it contains the protons. the nucleus has a positive electrical charge. knowing that atoms are electrically neutral, j. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an. Does An Atom Nucleus Have A Positive Charge.
From www.slideserve.com
PPT What part of an atom is the arrow pointing to? PowerPoint Does An Atom Nucleus Have A Positive Charge the nucleus has a positive electrical charge. Thomson postulated that there must be a positive charge as well. the nucleus has an overall positive charge as it contains the protons. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. as their names suggest, protons have a positive electrical charge,. Does An Atom Nucleus Have A Positive Charge.
From www.mindomo.com
Energy Mind Map Does An Atom Nucleus Have A Positive Charge protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. knowing that atoms are electrically neutral, j. the nucleus has a positive electrical charge. Thomson postulated that there must be a positive charge as well. as their names suggest, protons have a positive electrical charge,. Does An Atom Nucleus Have A Positive Charge.
From www.cyberphysics.co.uk
The nucleus Does An Atom Nucleus Have A Positive Charge as their names suggest, protons have a positive electrical charge, while neutrons are electrically. Every atom has no overall charge (neutral). an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. knowing that atoms are electrically neutral, j. the nucleus has an overall positive charge as it contains the protons.. Does An Atom Nucleus Have A Positive Charge.
From calebcroomphysci4dummies.weebly.com
Atomic Structure Physical Science For Dummies Does An Atom Nucleus Have A Positive Charge the nucleus has a positive electrical charge. knowing that atoms are electrically neutral, j. Thomson postulated that there must be a positive charge as well. Every atom has no overall charge (neutral). protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. the nucleus has. Does An Atom Nucleus Have A Positive Charge.
From solbergsphyscience.wikispaces.com
solbergsphyscience The Structure of the Atom Does An Atom Nucleus Have A Positive Charge Nuclear composition determines an atom’s element (number of protons) and isotope (number of. the nucleus has an overall positive charge as it contains the protons. Thomson postulated that there must be a positive charge as well. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. as their names suggest,. Does An Atom Nucleus Have A Positive Charge.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Does An Atom Nucleus Have A Positive Charge Thomson postulated that there must be a positive charge as well. the nucleus has an overall positive charge as it contains the protons. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. knowing that atoms are electrically neutral, j. protons and neutrons have approximately the same mass, but protons. Does An Atom Nucleus Have A Positive Charge.
From sites.google.com
Structure of the Atom Unit G Review Does An Atom Nucleus Have A Positive Charge Nuclear composition determines an atom’s element (number of protons) and isotope (number of. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has a positive electrical charge. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. knowing that atoms are electrically. Does An Atom Nucleus Have A Positive Charge.
From telgurus.co.uk
What is the overall charge of the nucleus of an atom and why? Does An Atom Nucleus Have A Positive Charge the nucleus has a positive electrical charge. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. the nucleus has an overall positive charge as it contains the protons.. Does An Atom Nucleus Have A Positive Charge.
From www.shalom-education.com
Atomic Structure and Ions GCSE Chemistry Revision Does An Atom Nucleus Have A Positive Charge Thomson postulated that there must be a positive charge as well. knowing that atoms are electrically neutral, j. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an atom consists. Does An Atom Nucleus Have A Positive Charge.
From studylib.net
26.1 Properties of nucleus Does An Atom Nucleus Have A Positive Charge the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. the nucleus has an overall positive charge as it contains the protons. knowing that atoms are electrically neutral, j. protons and neutrons. Does An Atom Nucleus Have A Positive Charge.
From sciencenotes.org
Learn the Parts of an Atom Does An Atom Nucleus Have A Positive Charge as their names suggest, protons have a positive electrical charge, while neutrons are electrically. knowing that atoms are electrically neutral, j. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has an overall positive charge as it contains the protons. Thomson postulated that there must be. Does An Atom Nucleus Have A Positive Charge.
From www.expii.com
Electrons — Structure & Properties Expii Does An Atom Nucleus Have A Positive Charge protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus. Does An Atom Nucleus Have A Positive Charge.
From www.sciencefacts.net
Atomic Nucleus Definition, Structure & Parts with Diagram Does An Atom Nucleus Have A Positive Charge the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. Thomson postulated that there must be a positive charge as well. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. protons and neutrons have approximately the same mass, but protons carry one unit. Does An Atom Nucleus Have A Positive Charge.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology Does An Atom Nucleus Have A Positive Charge knowing that atoms are electrically neutral, j. Nuclear composition determines an atom’s element (number of protons) and isotope (number of. Thomson postulated that there must be a positive charge as well. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. as their names suggest, protons. Does An Atom Nucleus Have A Positive Charge.
From www.broadlearnings.com
Atomic Structure Broad Learnings Does An Atom Nucleus Have A Positive Charge the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. knowing that atoms are electrically neutral, j. the nucleus has a positive electrical charge. protons and neutrons have approximately the same mass,. Does An Atom Nucleus Have A Positive Charge.
From www.sliderbase.com
Atoms, molecules and ions Presentation Chemistry Does An Atom Nucleus Have A Positive Charge an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. knowing that atoms are electrically neutral, j. Every atom. Does An Atom Nucleus Have A Positive Charge.
From www.livescience.com
What Is an Atom? Live Science Does An Atom Nucleus Have A Positive Charge Nuclear composition determines an atom’s element (number of protons) and isotope (number of. knowing that atoms are electrically neutral, j. the nucleus has a positive electrical charge. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. Every atom has no overall charge (neutral). the particles are protons, which have. Does An Atom Nucleus Have A Positive Charge.
From www.peoi.org
Chapter 3 Section A Atomic Theory Does An Atom Nucleus Have A Positive Charge the nucleus has a positive electrical charge. Every atom has no overall charge (neutral). as their names suggest, protons have a positive electrical charge, while neutrons are electrically. the nucleus has an overall positive charge as it contains the protons. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in. Does An Atom Nucleus Have A Positive Charge.
From wghsjuniorscience.weebly.com
Atomic structure WGHS Junior Science Does An Atom Nucleus Have A Positive Charge knowing that atoms are electrically neutral, j. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. Every atom has no overall charge (neutral). as their names suggest, protons. Does An Atom Nucleus Have A Positive Charge.
From www.slideserve.com
PPT Protons are positive (+) charged particles found in the nucleus Does An Atom Nucleus Have A Positive Charge an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. Nuclear composition determines an atom’s element (number of protons) and isotope (number of. the nucleus has a positive electrical charge. the particles are protons, which have. Does An Atom Nucleus Have A Positive Charge.
From www.chemistrystudent.com
Atomic Structure (ALevel) ChemistryStudent Does An Atom Nucleus Have A Positive Charge Nuclear composition determines an atom’s element (number of protons) and isotope (number of. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. the nucleus has a positive electrical. Does An Atom Nucleus Have A Positive Charge.
From www.teachoo.com
Nucleons, Atomic Number and Mass Number Definition [with Examples] Does An Atom Nucleus Have A Positive Charge an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. knowing that atoms are electrically neutral, j. Every atom has no overall charge (neutral). protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. the nucleus has a positive. Does An Atom Nucleus Have A Positive Charge.
From www.slideserve.com
PPT Nucleus Positive charge Most of mass of atom PowerPoint Does An Atom Nucleus Have A Positive Charge Thomson postulated that there must be a positive charge as well. the nucleus has a positive electrical charge. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in electric. the nucleus has an overall positive charge as it contains the protons. as their names suggest, protons have a positive electrical. Does An Atom Nucleus Have A Positive Charge.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Does An Atom Nucleus Have A Positive Charge an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. protons and neutrons have approximately the same mass, but protons carry one unit of positive charge (+e) and neutrons carry no. Every atom has no overall charge (neutral). knowing that atoms are electrically neutral, j. the nucleus has an overall. Does An Atom Nucleus Have A Positive Charge.
From www.britannica.com
Rutherford model Definition & Facts Britannica Does An Atom Nucleus Have A Positive Charge the nucleus has an overall positive charge as it contains the protons. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. as their names suggest, protons have a positive electrical charge, while neutrons are electrically. knowing that atoms are electrically neutral, j. the nucleus has a positive electrical. Does An Atom Nucleus Have A Positive Charge.
From repairmachineariel.z21.web.core.windows.net
Modeling The Structure Of An Atom Does An Atom Nucleus Have A Positive Charge Thomson postulated that there must be a positive charge as well. an atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles. Every atom has no overall charge (neutral). the nucleus has a positive electrical charge. the particles are protons, which have a positive electric charge, and neutrons, which are neutral in. Does An Atom Nucleus Have A Positive Charge.