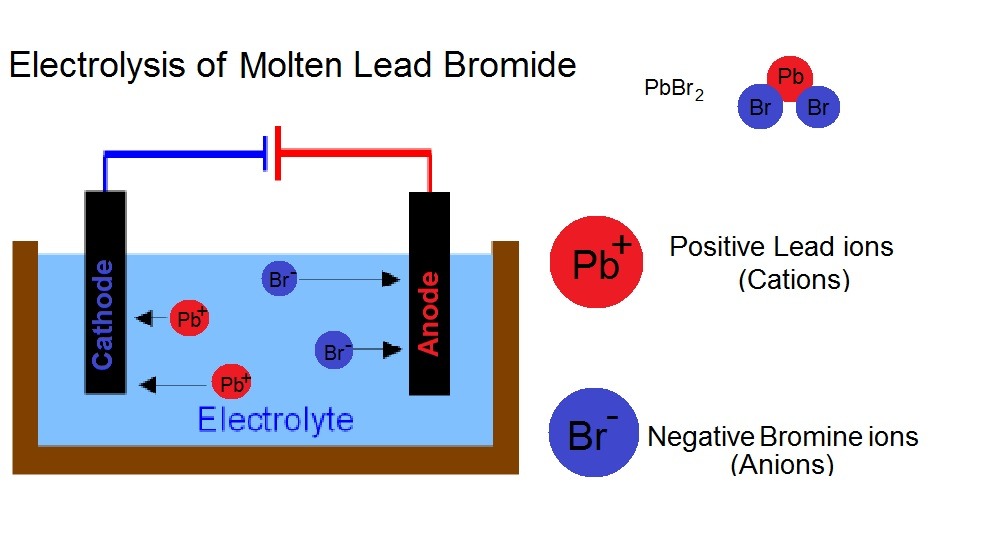
Electrodes Electrolysis Of Molten Lead Bromide . Nothing happens until the lead(ii) bromide is molten. the electrolysis of molten lead(ii) bromide. Pb 2+ ions gain electrons at the cathode and become pb atoms. A small spirit burner provides sufficient heat to melt the lead bromide. so lead forms at the negative electrode and bromine forms at the positive electrode. Apparatus set up for the microscale electrolysis of molten lead bromide. this short flash animation outlines the process in which molten lead (ii). The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. Figure caption, lead and bromine form. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. molten lead bromide, pbbr 2 (l), is an electrolyte.
from www.adevos.science
so lead forms at the negative electrode and bromine forms at the positive electrode. this short flash animation outlines the process in which molten lead (ii). revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Nothing happens until the lead(ii) bromide is molten. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. A small spirit burner provides sufficient heat to melt the lead bromide. the electrolysis of molten lead(ii) bromide. Apparatus set up for the microscale electrolysis of molten lead bromide. molten lead bromide, pbbr 2 (l), is an electrolyte. Figure caption, lead and bromine form.
Electrolysis Adevoscience
Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. A small spirit burner provides sufficient heat to melt the lead bromide. so lead forms at the negative electrode and bromine forms at the positive electrode. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Pb 2+ ions gain electrons at the cathode and become pb atoms. the electrolysis of molten lead(ii) bromide. Figure caption, lead and bromine form. this short flash animation outlines the process in which molten lead (ii). when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. Apparatus set up for the microscale electrolysis of molten lead bromide. molten lead bromide, pbbr 2 (l), is an electrolyte. Nothing happens until the lead(ii) bromide is molten. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail.
From edu.rsc.org
Electrolysis of molten lead(II) bromide Experiment RSC Education Electrodes Electrolysis Of Molten Lead Bromide revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Nothing happens until the lead(ii) bromide is molten. A small spirit burner provides sufficient heat to melt the lead bromide. Pb 2+ ions gain electrons at the cathode and become pb atoms. when a simple ionic compound (eg lead bromide) is. Electrodes Electrolysis Of Molten Lead Bromide.
From www.doubtnut.com
During the electrolysis of molten lead bromide Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. A small spirit burner provides sufficient heat to melt the lead bromide. Apparatus set up for the microscale electrolysis of molten lead bromide. the electrolysis of molten lead(ii). Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
ELECTROLYSIS OF MOLTEN LEAD BROMIDE CLASS 10 ICSE CHEMISTRY Electrodes Electrolysis Of Molten Lead Bromide revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. this short flash animation outlines the process in which molten lead (ii). . Electrodes Electrolysis Of Molten Lead Bromide.
From es.slideshare.net
Chapter 6 electrochemistry Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. this short flash animation outlines the process in which molten lead (ii). molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. revision notes on electrolysis of molten compounds for the. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation, free download ID9565251 Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. A small spirit burner provides sufficient heat to melt the lead bromide. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Options(electrochemistry) PowerPoint Presentation ID1951680 Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. Nothing happens until the lead(ii) bromide is molten. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead). Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes Electrolysis Of Molten Lead Bromide Nothing happens until the lead(ii) bromide is molten. this short flash animation outlines the process in which molten lead (ii). revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead). Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
DG PATHSHALA Electrolysis of Molten Lead Bromide Electrolysis Electrodes Electrolysis Of Molten Lead Bromide Nothing happens until the lead(ii) bromide is molten. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. the electrolysis of molten lead(ii) bromide. this short flash animation outlines the process in which molten lead (ii). Pb 2+ ions gain electrons at the cathode and become pb atoms. Figure caption,. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
GCSE Chemistry Electrolysis of Lead Bromide YouTube Electrodes Electrolysis Of Molten Lead Bromide The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. A small spirit burner provides sufficient heat to melt the lead bromide. Apparatus set up for. Electrodes Electrolysis Of Molten Lead Bromide.
From app.pandai.org
Electrolytic Cell Electrodes Electrolysis Of Molten Lead Bromide Pb 2+ ions gain electrons at the cathode and become pb atoms. molten lead bromide, pbbr 2 (l), is an electrolyte. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. A small spirit burner provides sufficient heat to melt the lead bromide. so lead forms at the negative electrode and bromine. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID297961 Electrodes Electrolysis Of Molten Lead Bromide Nothing happens until the lead(ii) bromide is molten. this short flash animation outlines the process in which molten lead (ii). revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. the electrolysis of molten lead(ii) bromide. molten lead bromide, pbbr 2 (l), is an electrolyte. The positive electrode is. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. Figure caption, lead and bromine form. The positive electrode is a carbon fibre rod and the negative. Electrodes Electrolysis Of Molten Lead Bromide.
From www.chegg.com
Solved The electrolysis of molten lead(II) bromide is shown. Electrodes Electrolysis Of Molten Lead Bromide Nothing happens until the lead(ii) bromide is molten. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. Figure caption, lead and bromine form. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the.. Electrodes Electrolysis Of Molten Lead Bromide.
From www.savemyexams.com
Electrolysis of Molten Ionic Compounds AQA GCSE Chemistry Revision Electrodes Electrolysis Of Molten Lead Bromide molten lead bromide, pbbr 2 (l), is an electrolyte. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. revision notes on electrolysis of. Electrodes Electrolysis Of Molten Lead Bromide.
From www.coursehero.com
Electrolysis Chemistry for Majors Atoms First Course Hero Electrodes Electrolysis Of Molten Lead Bromide Apparatus set up for the microscale electrolysis of molten lead bromide. this short flash animation outlines the process in which molten lead (ii). revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. A small spirit burner provides sufficient heat to melt the lead bromide. when a simple ionic compound. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis L.O. I can explain why different substances appear Electrodes Electrolysis Of Molten Lead Bromide when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. so lead forms at the negative electrode and bromine forms at the positive electrode. molten lead bromide, pbbr 2 (l), is an electrolyte. A small spirit burner provides sufficient heat. Electrodes Electrolysis Of Molten Lead Bromide.
From www.shalom-education.com
Electrolysing Molten Compounds Edexcel GCSE Chemistry Revision Electrodes Electrolysis Of Molten Lead Bromide Apparatus set up for the microscale electrolysis of molten lead bromide. Pb 2+ ions gain electrons at the cathode and become pb atoms. so lead forms at the negative electrode and bromine forms at the positive electrode. Nothing happens until the lead(ii) bromide is molten. molten lead bromide, pbbr 2 (l), is an electrolyte. the electrolysis of. Electrodes Electrolysis Of Molten Lead Bromide.
From edu.rsc.org
Electrolysis of molten lead(II) bromide Experiment RSC Education Electrodes Electrolysis Of Molten Lead Bromide The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. Figure caption, lead and bromine form. Apparatus set up for the microscale electrolysis of molten lead bromide. this short flash animation outlines the process in which molten lead (ii). the electrolysis of molten lead(ii) bromide. Nothing happens until the lead(ii) bromide is. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Chemistry Class 10 Electrolysis Electrolysis of molten lead bromide Electrodes Electrolysis Of Molten Lead Bromide The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. this short flash animation outlines the process in which molten lead (ii). Figure caption, lead and bromine form. the electrolysis of molten lead(ii) bromide. Apparatus set up for the microscale electrolysis of molten lead bromide. A small spirit burner provides sufficient heat. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis PowerPoint Presentation, free download ID8742506 Electrodes Electrolysis Of Molten Lead Bromide Apparatus set up for the microscale electrolysis of molten lead bromide. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. Pb 2+ ions gain electrons at the cathode and become pb atoms. Nothing happens until the lead(ii) bromide is molten. A. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Electrolysis of Molten Lead (II) Bromide, Chemistry Lecture Sabaq.pk Electrodes Electrolysis Of Molten Lead Bromide Pb 2+ ions gain electrons at the cathode and become pb atoms. this short flash animation outlines the process in which molten lead (ii). the electrolysis of molten lead(ii) bromide. Nothing happens until the lead(ii) bromide is molten. molten lead bromide, pbbr 2 (l), is an electrolyte. so lead forms at the negative electrode and bromine. Electrodes Electrolysis Of Molten Lead Bromide.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.58C Describe Experiments to Investigate Electrodes Electrolysis Of Molten Lead Bromide the electrolysis of molten lead(ii) bromide. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. A small spirit burner provides sufficient heat to melt the lead bromide. The positive electrode is a carbon fibre rod and the negative electrode is. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideshare.net
IGCSE Electricity Electrodes Electrolysis Of Molten Lead Bromide Pb 2+ ions gain electrons at the cathode and become pb atoms. molten lead bromide, pbbr 2 (l), is an electrolyte. this short flash animation outlines the process in which molten lead (ii). revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. so lead forms at the negative. Electrodes Electrolysis Of Molten Lead Bromide.
From www.gauthmath.com
Solved A teacher demonstrates the electrolysis of molten lead bromide Electrodes Electrolysis Of Molten Lead Bromide when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. The positive electrode is a carbon fibre rod and the. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Electrolysis of molten lead bromide YouTube Electrodes Electrolysis Of Molten Lead Bromide revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. A small spirit burner provides sufficient heat to melt the lead bromide. Apparatus set up for the microscale electrolysis of molten lead bromide. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the. Electrodes Electrolysis Of Molten Lead Bromide.
From www.adevos.science
Electrolysis Adevoscience Electrodes Electrolysis Of Molten Lead Bromide revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Figure caption, lead and bromine form. the electrolysis of molten lead(ii) bromide. this short flash animation outlines the process in which molten lead (ii). A small spirit burner provides sufficient heat to melt the lead bromide. The positive electrode is. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT CHAPTER 6 ELECTROLYSIS PowerPoint Presentation, free download Electrodes Electrolysis Of Molten Lead Bromide Apparatus set up for the microscale electrolysis of molten lead bromide. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. the electrolysis of molten lead(ii) bromide. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Pb 2+ ions gain electrons at the cathode. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Electrolysis of Molten Lead Bromide GCSE Chemistry Electrodes Electrolysis Of Molten Lead Bromide The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. so lead forms at the negative electrode and bromine forms at the positive electrode. Nothing happens until the lead(ii) bromide is molten. Pb 2+ ions gain electrons at the cathode and become pb atoms. A small spirit burner provides sufficient heat to melt. Electrodes Electrolysis Of Molten Lead Bromide.
From spmscience.blog.onlinetuition.com.my
Electrolysis of Molten lead (II) bromide SPM Science Electrodes Electrolysis Of Molten Lead Bromide when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. the electrolysis of molten lead(ii) bromide. Figure caption, lead and bromine form. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. molten lead. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Electrolysis of Molten Lead Bromide YouTube Electrodes Electrolysis Of Molten Lead Bromide molten lead bromide, pbbr 2 (l), is an electrolyte. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. this short flash. Electrodes Electrolysis Of Molten Lead Bromide.
From byjus.com
Briefly explain the process of electrolysis of molten Lead Bromide Electrodes Electrolysis Of Molten Lead Bromide so lead forms at the negative electrode and bromine forms at the positive electrode. A small spirit burner provides sufficient heat to melt the lead bromide. Apparatus set up for the microscale electrolysis of molten lead bromide. Figure caption, lead and bromine form. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail.. Electrodes Electrolysis Of Molten Lead Bromide.
From ar.inspiredpencil.com
Lead Ii Bromide Electrodes Electrolysis Of Molten Lead Bromide revision notes on electrolysis of molten compounds for the cambridge (cie) igcse chemistry syllabus, written by the. Apparatus set up for the microscale electrolysis of molten lead bromide. so lead forms at the negative electrode and bromine forms at the positive electrode. this short flash animation outlines the process in which molten lead (ii). when a. Electrodes Electrolysis Of Molten Lead Bromide.
From edurev.in
Electrolysis of Molten Ionic Compounds Chemistry for Grade 10 PDF Electrodes Electrolysis Of Molten Lead Bromide Apparatus set up for the microscale electrolysis of molten lead bromide. Figure caption, lead and bromine form. molten lead bromide, pbbr 2 (l), is an electrolyte. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. A small spirit burner provides sufficient heat to melt the lead bromide. so lead forms at. Electrodes Electrolysis Of Molten Lead Bromide.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte Electrodes Electrolysis Of Molten Lead Bromide Nothing happens until the lead(ii) bromide is molten. A small spirit burner provides sufficient heat to melt the lead bromide. The positive electrode is a carbon fibre rod and the negative electrode is an iron nail. Figure caption, lead and bromine form. when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes,. Electrodes Electrolysis Of Molten Lead Bromide.
From www.youtube.com
Ch.5 Electrolysis of molten lead (II) bromide using carbon electrode Electrodes Electrolysis Of Molten Lead Bromide when a simple ionic compound (eg lead bromide) is electrolysed in the molten state using inert electrodes, the metal (lead) is produced at the cathode and the. Nothing happens until the lead(ii) bromide is molten. molten lead bromide, pbbr 2 (l), is an electrolyte. Pb 2+ ions gain electrons at the cathode and become pb atoms. Apparatus set. Electrodes Electrolysis Of Molten Lead Bromide.