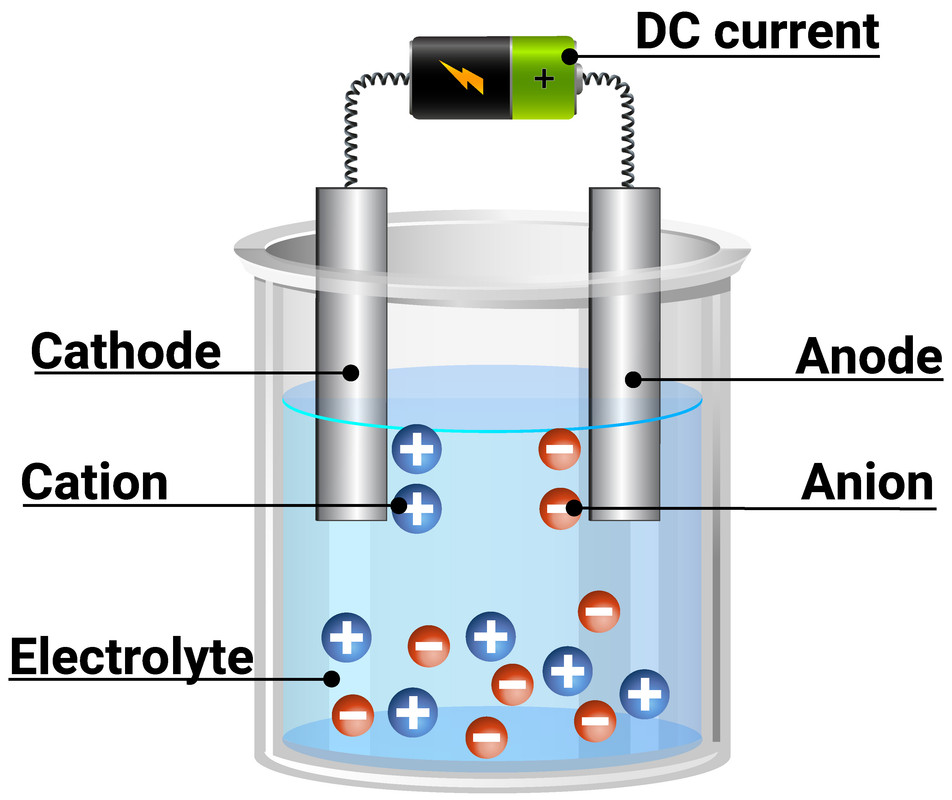
How Do Inert Electrodes Work . Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. How do inert electrodes contribute to the function of electrochemical cells? Inert electrodes play a critical role in electrochemical cells by providing. See examples of galvanic cells. See examples of galvanic cells with. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Find out how to measure and control the cell potential, and how to. Inert electrolyte solutions are used to balance charge and maintain salt bridges.
from www.revisechemistry.uk
Find out how to measure and control the cell potential, and how to. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. See examples of galvanic cells with. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical cells by providing. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. How do inert electrodes contribute to the function of electrochemical cells? See examples of galvanic cells.
Electrolysis OCR Gateway C3 revisechemistry.uk
How Do Inert Electrodes Work Find out how to measure and control the cell potential, and how to. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. See examples of galvanic cells with. See examples of galvanic cells. Find out how to measure and control the cell potential, and how to. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrolyte solutions are used to balance charge and maintain salt bridges. How do inert electrodes contribute to the function of electrochemical cells? Inert electrodes play a critical role in electrochemical cells by providing.
From www.youtube.com
Inert electrodes Trew’s notes YouTube How Do Inert Electrodes Work Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical cells by providing. How do. How Do Inert Electrodes Work.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical. How Do Inert Electrodes Work.
From www.youtube.com
S17E3 Inert Electrodes, and Galvanic Cell Practice Problems YouTube How Do Inert Electrodes Work See examples of galvanic cells. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrodes play a critical role in electrochemical cells by providing. Find out how to measure and control the cell potential, and how to. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo. How Do Inert Electrodes Work.
From www.slideserve.com
PPT ELECTROLYSIS PowerPoint Presentation ID4966899 How Do Inert Electrodes Work See examples of galvanic cells with. See examples of galvanic cells. Find out how to measure and control the cell potential, and how to. Inert electrodes play a critical role in electrochemical cells by providing. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Learn how to build and use electrochemical. How Do Inert Electrodes Work.
From courses.lumenlearning.com
Electrolysis Chemistry Atoms First How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. See examples of galvanic cells with. Find out how to measure and control the cell potential, and how to. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Inert electrodes play a critical role. How Do Inert Electrodes Work.
From www.youtube.com
Electrolysis of dilute sodium chloride (inert electrodes) YouTube How Do Inert Electrodes Work Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. How do inert electrodes contribute to the function of electrochemical. How Do Inert Electrodes Work.
From saylordotorg.github.io
Electrochemistry How Do Inert Electrodes Work See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Find out how to measure and control the cell potential, and how to. Learn how galvanic cells use spontaneous redox reactions to. How Do Inert Electrodes Work.
From exopopjam.blob.core.windows.net
Electrode Examples at Hillary Browning blog How Do Inert Electrodes Work See examples of galvanic cells. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. See examples of galvanic cells with. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. How do inert electrodes contribute to the function of electrochemical. How Do Inert Electrodes Work.
From chem.libretexts.org
17.3 Standard Reduction Potentials Chemistry LibreTexts How Do Inert Electrodes Work Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? Inert electrodes play a. How Do Inert Electrodes Work.
From loegolswy.blob.core.windows.net
What Does Inert Electrodes Mean at Connie Guess blog How Do Inert Electrodes Work See examples of galvanic cells. See examples of galvanic cells with. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrodes play a critical role in electrochemical cells by providing. Find out how to measure and control the cell potential, and how to. Learn how to build and use electrochemical cells,. How Do Inert Electrodes Work.
From exopopjam.blob.core.windows.net
Electrode Examples at Hillary Browning blog How Do Inert Electrodes Work See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrolyte solutions are used. How Do Inert Electrodes Work.
From www.chemistry-teaching-resources.com
chemistry picture How Do Inert Electrodes Work Find out how to measure and control the cell potential, and how to. How do inert electrodes contribute to the function of electrochemical cells? Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical cells by providing. See examples of galvanic cells with. Learn how galvanic cells use spontaneous redox. How Do Inert Electrodes Work.
From loegolswy.blob.core.windows.net
What Does Inert Electrodes Mean at Connie Guess blog How Do Inert Electrodes Work Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. See examples of galvanic cells. Inert electrodes play a critical role in electrochemical cells by providing. Find out how to measure and control the cell potential, and how to. How do inert electrodes contribute to the function of electrochemical. How Do Inert Electrodes Work.
From exotsdtks.blob.core.windows.net
Electrolysis Electrodes Reaction at Leroy Russell blog How Do Inert Electrodes Work See examples of galvanic cells with. Find out how to measure and control the cell potential, and how to. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical. How Do Inert Electrodes Work.
From www.researchgate.net
Schematic illustration of a typical three‐electrode system. Download How Do Inert Electrodes Work Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Find out how to measure and control the cell potential,. How Do Inert Electrodes Work.
From loegolswy.blob.core.windows.net
What Does Inert Electrodes Mean at Connie Guess blog How Do Inert Electrodes Work See examples of galvanic cells. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrodes play a critical role in electrochemical cells by providing. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. How do inert electrodes contribute to. How Do Inert Electrodes Work.
From semcouniversity.com
How the three electrode system works Semco University Semco How Do Inert Electrodes Work Inert electrodes play a critical role in electrochemical cells by providing. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Find out how to measure and control the cell potential, and how to. See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? Learn how. How Do Inert Electrodes Work.
From icsechemistry16.blogspot.com
Electrolysis of Copper sulphate using inert electrodes How Do Inert Electrodes Work Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. See examples of galvanic cells. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical cells by providing. See examples of galvanic cells with. How do inert electrodes contribute to the function. How Do Inert Electrodes Work.
From www.kovinc.com
TIG Welding Tungsten Inert Gas Welding Kovinc d.o.o. How Do Inert Electrodes Work Find out how to measure and control the cell potential, and how to. Inert electrodes play a critical role in electrochemical cells by providing. See examples of galvanic cells. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. See examples of galvanic cells with. Learn how galvanic cells. How Do Inert Electrodes Work.
From courses.lumenlearning.com
17.2 Galvanic Cells Chemistry How Do Inert Electrodes Work See examples of galvanic cells with. Inert electrodes play a critical role in electrochemical cells by providing. How do inert electrodes contribute to the function of electrochemical cells? See examples of galvanic cells. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Learn about galvanic cells, which consist of two electrodes in. How Do Inert Electrodes Work.
From www.youtube.com
Ch. 203 Inert Electrodes/Voltaic Cells YouTube How Do Inert Electrodes Work See examples of galvanic cells. Inert electrodes play a critical role in electrochemical cells by providing. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to. How Do Inert Electrodes Work.
From loegolswy.blob.core.windows.net
What Does Inert Electrodes Mean at Connie Guess blog How Do Inert Electrodes Work Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. See examples of galvanic cells with. See examples of galvanic cells. How do inert electrodes contribute to the function of electrochemical cells? Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn how galvanic cells use spontaneous redox reactions. How Do Inert Electrodes Work.
From saylordotorg.github.io
Electrochemistry How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? See examples of galvanic cells. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Find out how. How Do Inert Electrodes Work.
From www.youtube.com
Inert electrodes YouTube How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrolyte solutions are used to balance charge and maintain salt. How Do Inert Electrodes Work.
From chem.libretexts.org
Standard Potentials Chemistry LibreTexts How Do Inert Electrodes Work See examples of galvanic cells. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn how galvanic cells. How Do Inert Electrodes Work.
From www.youtube.com
Electrolysis inert electrode O level chemistry by Ms Chew YouTube How Do Inert Electrodes Work Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrodes play a critical role in electrochemical cells by providing. How do inert electrodes contribute to the function of electrochemical cells?. How Do Inert Electrodes Work.
From www.slideserve.com
PPT ELECTROCHEMISTRY PowerPoint Presentation, free download ID6102398 How Do Inert Electrodes Work See examples of galvanic cells with. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Inert electrodes play a critical role in electrochemical cells by providing. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Learn how to build and use electrochemical cells, which are devices that convert. How Do Inert Electrodes Work.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk How Do Inert Electrodes Work See examples of galvanic cells with. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Find out how to measure and control the cell potential, and how to. See examples of galvanic cells. Inert electrodes play a critical role in electrochemical cells by providing. Learn how to build and use electrochemical. How Do Inert Electrodes Work.
From www.youtube.com
Chemistry Electrolysis (Inert & Active Electrodes) YouTube How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Inert electrodes play a critical role in electrochemical cells by providing. See examples. How Do Inert Electrodes Work.
From quizunbestowed.z21.web.core.windows.net
Why Are Inert Electrodes Used In Electrolysis How Do Inert Electrodes Work Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Inert electrodes play a critical role in electrochemical cells by providing. See examples of galvanic cells with. How do inert electrodes contribute to the function of electrochemical cells? Inert electrolyte solutions are used to balance charge and maintain salt bridges. See examples of. How Do Inert Electrodes Work.
From www.youtube.com
Ch31 part 1 chemical cell with inert electrodes YouTube How Do Inert Electrodes Work See examples of galvanic cells with. Learn about galvanic cells, which consist of two electrodes in different solutions that undergo oxidation and reduction reactions. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrolyte solutions are used to balance charge and maintain salt bridges. See examples of. How Do Inert Electrodes Work.
From brainly.in
There are two types of electrodes and they are Active electrode Inert How Do Inert Electrodes Work Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Find out how to measure and control the cell potential, and how to. Inert electrodes play a critical role in electrochemical cells by providing. Inert electrolyte solutions are used to balance charge and maintain salt bridges. See examples of. How Do Inert Electrodes Work.
From courses.lumenlearning.com
Galvanic Cells Chemistry How Do Inert Electrodes Work Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Learn how galvanic cells use spontaneous redox reactions to generate electricity and how to write cell notations. Find out how to measure and control the cell potential, and how to. Learn about galvanic cells, which consist of two electrodes. How Do Inert Electrodes Work.
From www.slideserve.com
PPT electrolysis of solutions PowerPoint Presentation, free download How Do Inert Electrodes Work See examples of galvanic cells. Inert electrolyte solutions are used to balance charge and maintain salt bridges. How do inert electrodes contribute to the function of electrochemical cells? Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrodes play a critical role in electrochemical cells by providing.. How Do Inert Electrodes Work.
From www.slideserve.com
PPT REDOX EQM Half Cells with Inert Electrode PowerPoint Presentation How Do Inert Electrodes Work How do inert electrodes contribute to the function of electrochemical cells? Find out how to measure and control the cell potential, and how to. Learn how to build and use electrochemical cells, which are devices that convert chemical energy into electrical energy or vice versa. Inert electrolyte solutions are used to balance charge and maintain salt bridges. Learn how galvanic. How Do Inert Electrodes Work.